The Nature of Light Honors l Dual Nature










- Slides: 31

The Nature of Light (Honors) l Dual Nature of Light: l behaves as both waves and as particles (depending on what type of experiment is being performed. ) l Speed of Light: light waves travel at same velocity l C = 3. 0 x 108 meters/sec l l What is Light? https: //www. youtube. com/watch? v=e. CVPhj. Hh 57 E Greatest Discovery in Physics: (Duality of Light) https: // www. youtube. com/watch? v=XB-i. LRsq 8 A 8 https: //www. youtube. com/watch? v=XB-i. LRsq 8 A 8

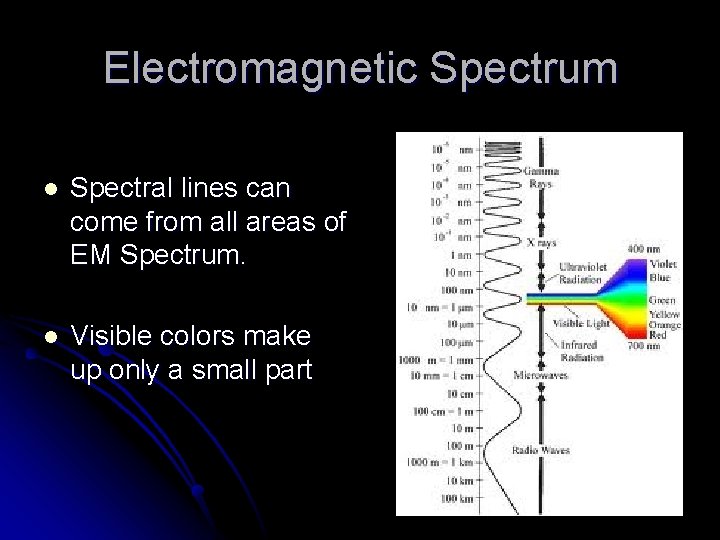
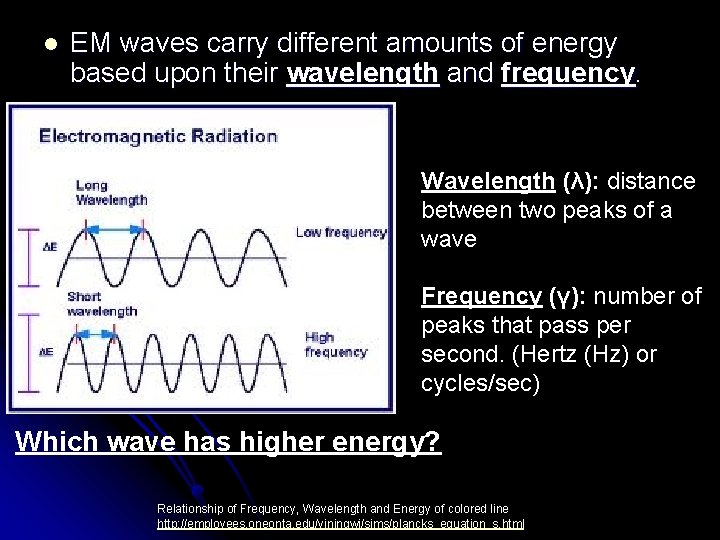
Electromagnetic Spectrum l Spectral lines can come from all areas of EM Spectrum. l Visible colors make up only a small part

l EM waves carry different amounts of energy based upon their wavelength and frequency. Wavelength (λ): distance between two peaks of a wave Frequency (γ): number of peaks that pass per second. (Hertz (Hz) or cycles/sec) Which wave has higher energy? Relationship of Frequency, Wavelength and Energy of colored line http: //employees. oneonta. edu/viningwj/sims/plancks_equation_s. html

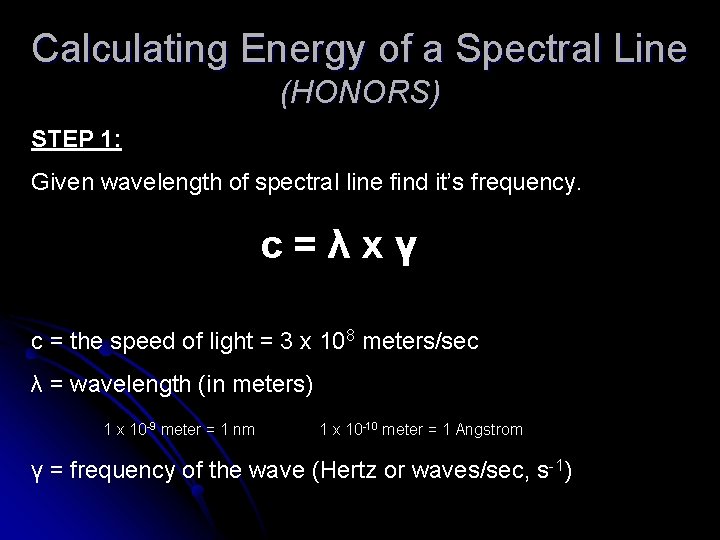
Calculating Energy of a Spectral Line (HONORS) STEP 1: Given wavelength of spectral line find it’s frequency. c=λxү c = the speed of light = 3 x 108 meters/sec λ = wavelength (in meters) 1 x 10 -9 meter = 1 nm 1 x 10 -10 meter = 1 Angstrom ү = frequency of the wave (Hertz or waves/sec, s-1)

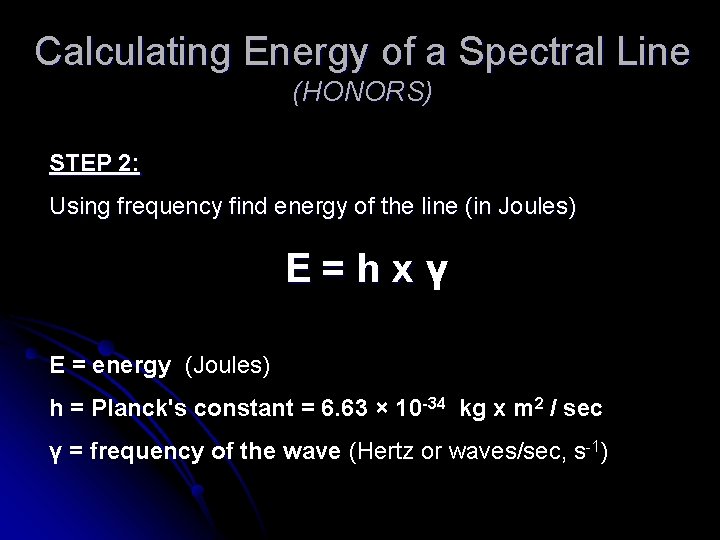
Calculating Energy of a Spectral Line (HONORS) STEP 2: Using frequency find energy of the line (in Joules) E=hxү E = energy (Joules) h = Planck's constant = 6. 63 × 10 -34 kg x m 2 / sec ү = frequency of the wave (Hertz or waves/sec, s-1)


How are Electrons Organized? Energy Levels Sublevels Orbitals Spin The Electron Hotel

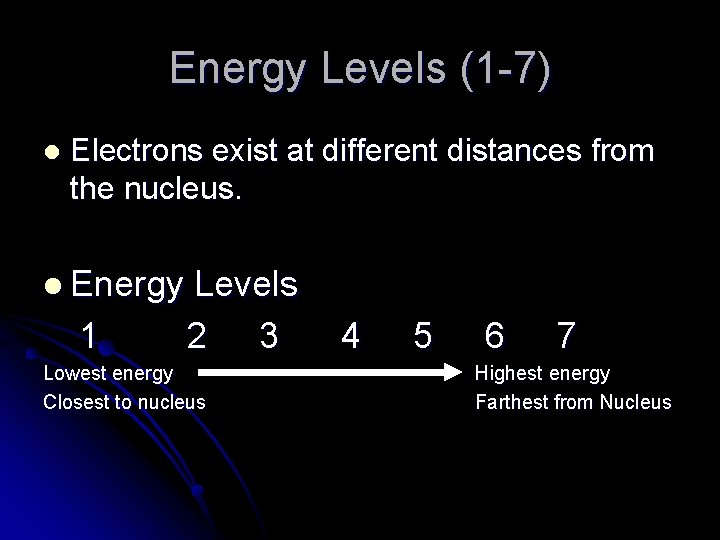
Energy Levels (1 -7) l Electrons exist at different distances from the nucleus. l Energy 1 Levels 2 3 Lowest energy Closest to nucleus 4 5 6 7 Highest energy Farthest from Nucleus

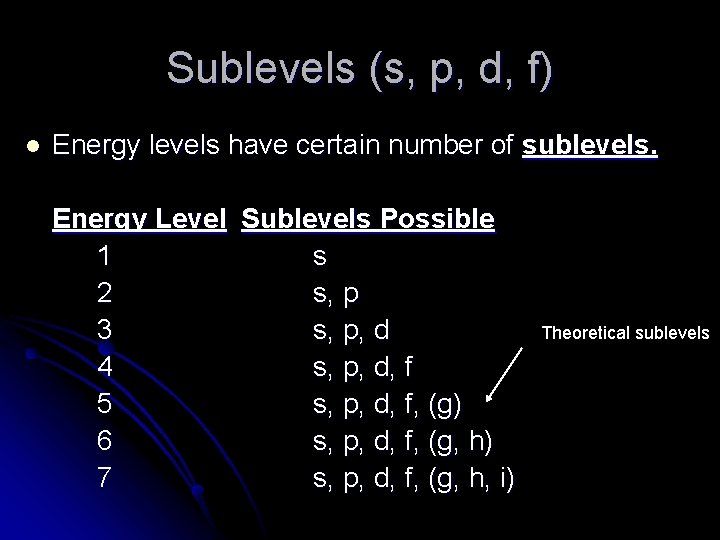
Sublevels (s, p, d, f) l Energy levels have certain number of sublevels. Energy Level Sublevels Possible 1 s 2 s, p 3 s, p, d 4 s, p, d, f 5 s, p, d, f, (g) 6 s, p, d, f, (g, h) 7 s, p, d, f, (g, h, i) Theoretical sublevels

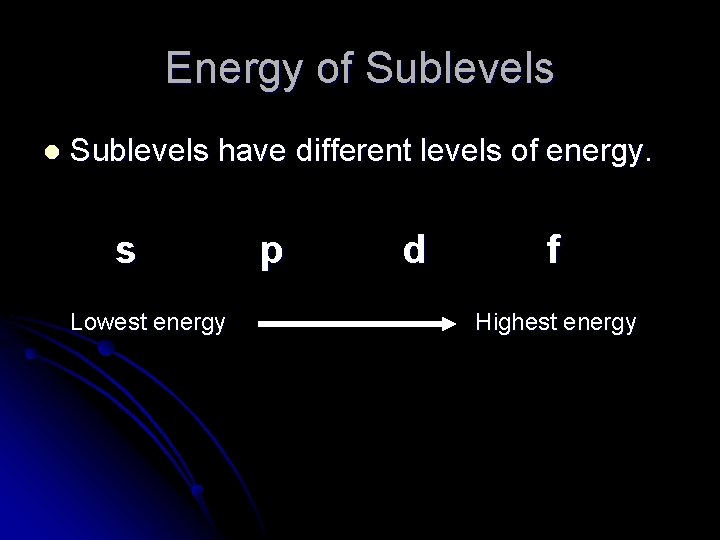
Energy of Sublevels l Sublevels have different levels of energy. s Lowest energy p d f Highest energy

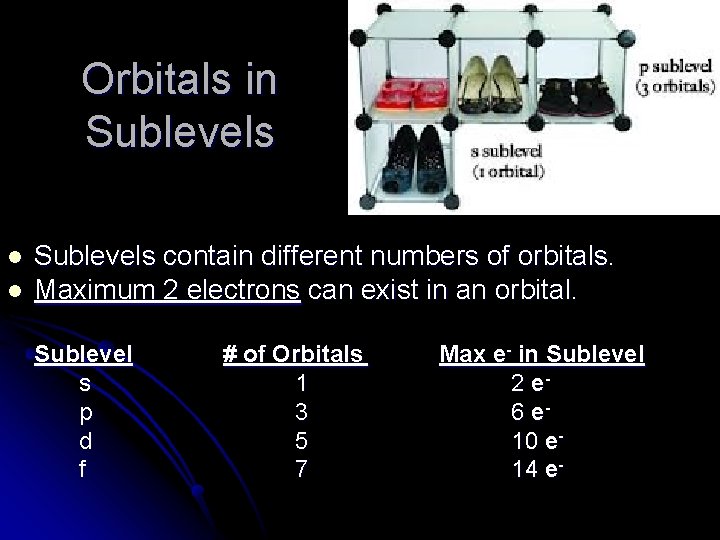
Orbitals in Sublevels l l Sublevels contain different numbers of orbitals. Maximum 2 electrons can exist in an orbital. Sublevel s p d f # of Orbitals 1 3 5 7 Max e- in Sublevel 2 e 6 e 10 e 14 e-

Aufbau Diagram l Shows: l order of electron filling l order of electron energy l Follow the “diagonal rule”

Writing Electron Configurations l Let’s write some electron configurations! l Ex: Magnesium l Follow the diagonal rule

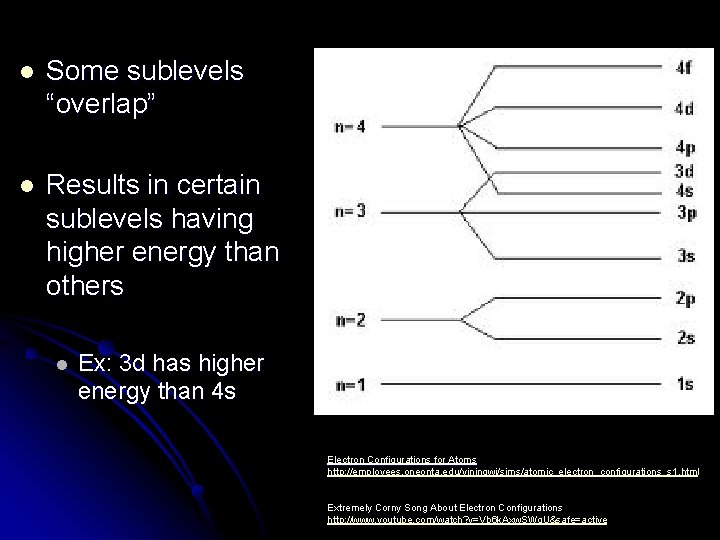
l Some sublevels “overlap” l Results in certain sublevels having higher energy than others l Ex: 3 d has higher energy than 4 s Electron Configurations for Atoms http: //employees. oneonta. edu/viningwj/sims/atomic_electron_configurations_s 1. html Extremely Corny Song About Electron Configurations http: //www. youtube. com/watch? v=Vb 6 k. Axw. SWg. U&safe=active

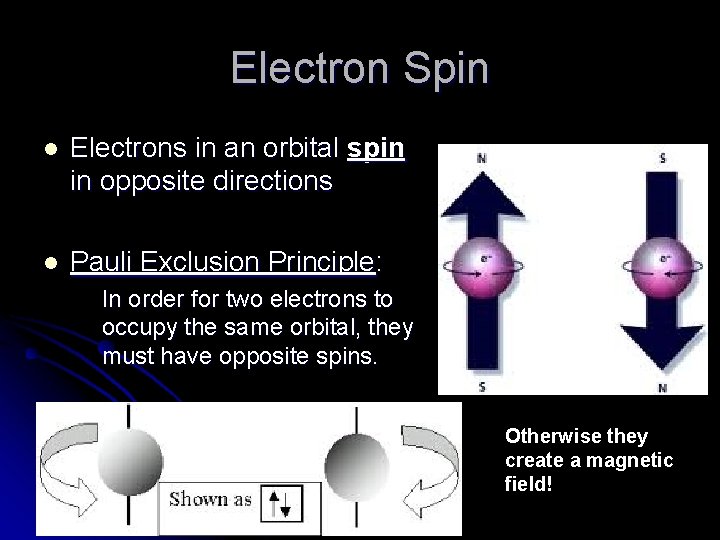
Electron Spin l Electrons in an orbital spin in opposite directions l Pauli Exclusion Principle: In order for two electrons to occupy the same orbital, they must have opposite spins. Otherwise they create a magnetic field!

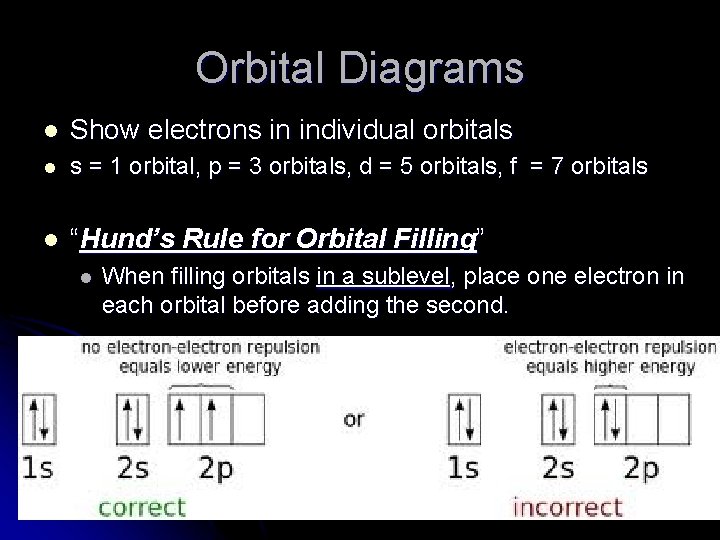
Orbital Diagrams l Show electrons in individual orbitals l s = 1 orbital, p = 3 orbitals, d = 5 orbitals, f = 7 orbitals l “Hund’s Rule for Orbital Filling” l When filling orbitals in a sublevel, place one electron in each orbital before adding the second.


Shapes of Orbitals l Orbitals come in different shapes and sizes. l Region of highest probability of finding an electron. l l Orbitals Shape & Energy & Spectral Line http: //www. youtube. com/watch? v=f. KYso 97 e. Js 4

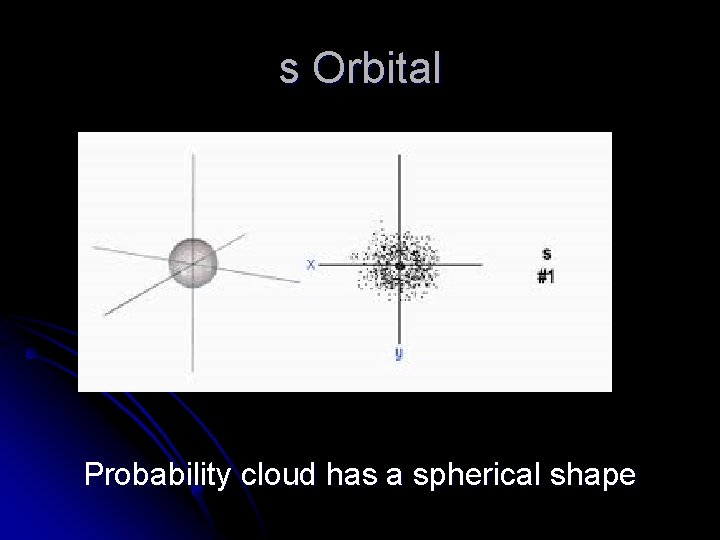
s Orbital Probability cloud has a spherical shape

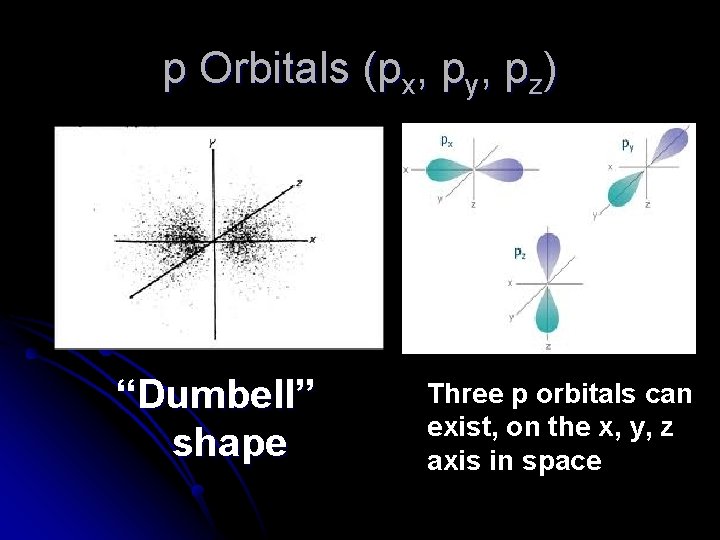
p Orbitals (px, py, pz) “Dumbell” shape Three p orbitals can exist, on the x, y, z axis in space

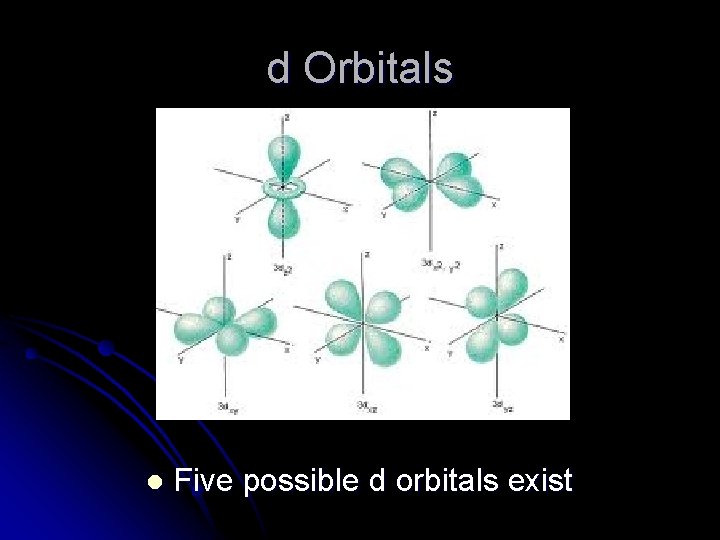
d Orbitals l Five possible d orbitals exist

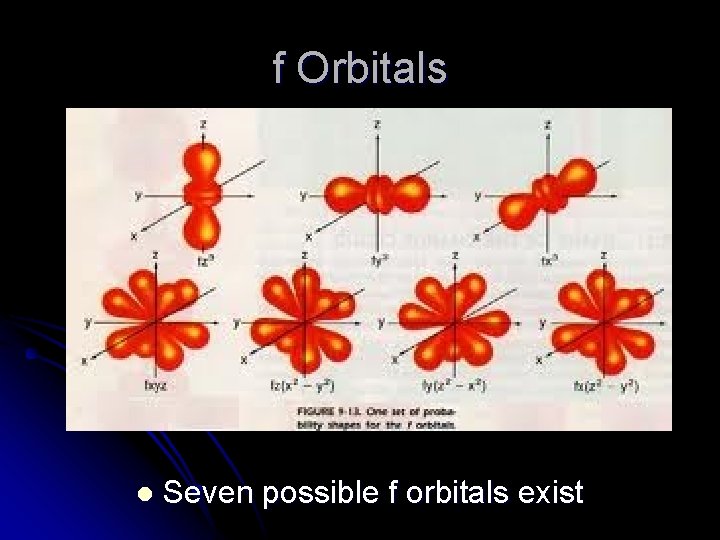
f Orbitals l Seven possible f orbitals exist

Valence Electrons l Usually found in the s and p sublevels of highest occupied energy level. l How many valence electrons? l Draw a Lewis Dot Diagram of this element. l 1 s 22 p 63 s 23 p 64 s 23 d 104 p 2

Kernel l All electrons except the valence 1 s 22 p 63 s 23 p 3 l How many kernel electrons? l How many valence?

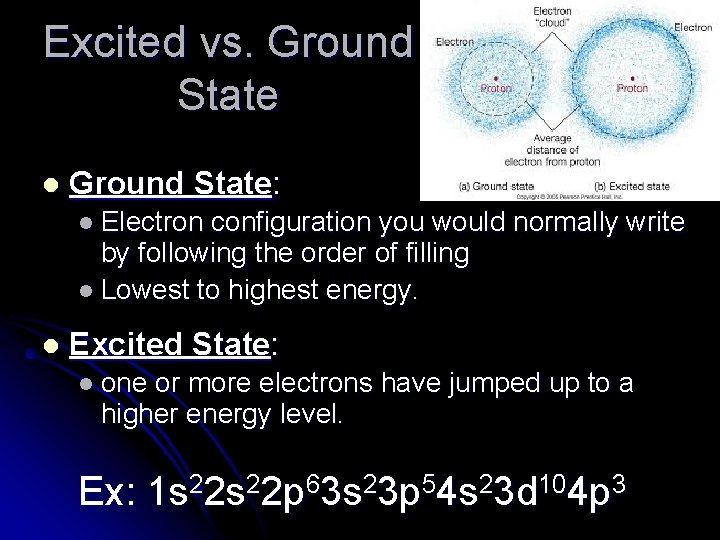
Excited vs. Ground State l Ground State: l Electron configuration you would normally write by following the order of filling l Lowest to highest energy. l Excited State: l one or more electrons have jumped up to a higher energy level. Ex: 1 s 22 p 63 s 23 p 54 s 23 d 104 p 3

Atom vs. Ion Configurations l Ions: atoms that have gained or lost electrons. l Figure out how many electrons the ion has then write configuration. l Ex: 20 Ca+2 has 18 electrons l 1 s 22 p 63 s 23 p 6 = 18 electrons Electron Configurations for Ions http: //employees. oneonta. edu/viningwj/sims/atomic_electron_configurations_s 2. html DONE!

Isoelectronic Species l Atoms and ions that have the same number of electrons. l Ex: Ar, K+1, Ca+2, P-3, O-2, Cl-1 l All have 18 electrons!

Impossible Configurations l Break the rules. Ex: 1 s 22 p 63 s 22 d 103 p 64 s 23 d 104 p 2 Ex: 1 s 22 s 32 p 63 s 23 p 6

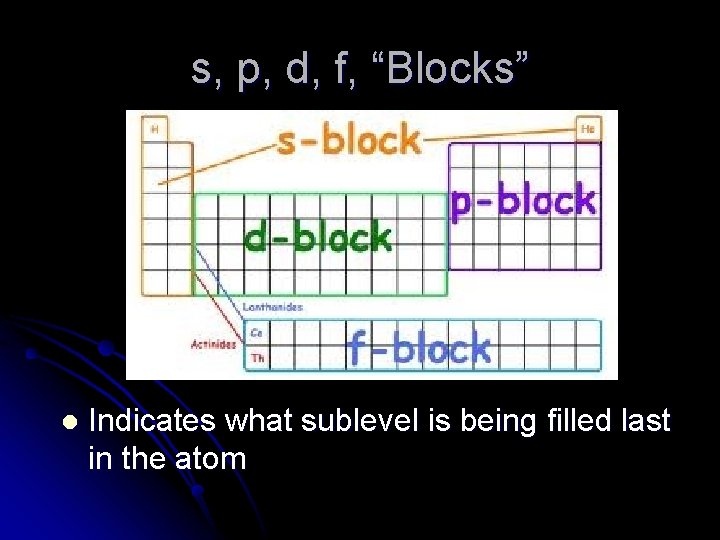
s, p, d, f, “Blocks” l Indicates what sublevel is being filled last in the atom

Some Exceptions to Orbital Filling (HONORS) l When d and f sublevels get filled near the end of a configuration we sometimes see exceptions. l It is more stable for the orbitals of the d and f sublevels to be half filled or filled completely than to be one shy. l Electrons from the sublevel below get “promoted” up to make the atom more stable l Ex: Copper

Crash Course Chemistry: The Electron (13 minutes) l http: //www. youtube. com/watch? v=rc. Kil. E 9 Cda. A l l Quantum Mechanics and the Bohr Model (6 minutes) http: //www. youtube. com/watch? v=-YYBCNQn. YNM **Developing Modern Atomic Theory (6 min) Rutherford to Bohr to the Modern Model http: //youtu. be/45 KGS 1 Ro-sc The Uncertainty Principle (6 min) Honors Probability & Chance of Finding an e- and Orbital Shapes http: //www. youtube. com/watch? v=Fw 6 d. I 7 cgu. Cg
 Thompson
Thompson Light has a dual nature
Light has a dual nature Dual nature of light
Dual nature of light Photon
Photon Dual nature of light
Dual nature of light Mirror formula
Mirror formula Light light light chapter 23
Light light light chapter 23 Light light light chapter 22
Light light light chapter 22 Light light light chapter 22
Light light light chapter 22 X factor
X factor Pathfinder shooting star
Pathfinder shooting star Ucsb frap
Ucsb frap Honors chemistry summer assignment
Honors chemistry summer assignment Tulane honors program
Tulane honors program Honors earth science
Honors earth science Honors algebra 2 final exam
Honors algebra 2 final exam Honors biology unit 4 test
Honors biology unit 4 test Creighton honors program
Creighton honors program English 2 honors vocabulary unit 1
English 2 honors vocabulary unit 1 Honors project
Honors project Honors biology ecology test
Honors biology ecology test Physics honors notes
Physics honors notes Algebra 1 honors
Algebra 1 honors Physics semester 1 review
Physics semester 1 review Chapter 3 geometry test
Chapter 3 geometry test Kuei honors chemistry
Kuei honors chemistry Umes honors program
Umes honors program James scholar uiuc aces
James scholar uiuc aces Math 3 honors
Math 3 honors Honors biology properties of water lab
Honors biology properties of water lab 04.05 uncle sams toolbox-honors
04.05 uncle sams toolbox-honors Mahurin honors college
Mahurin honors college