The costeffectiveness of needle and syringe provision in






- Slides: 27

The cost-effectiveness of needle and syringe provision in preventing transmission of Hepatitis C Virus in people who inject drugs in the UK Zoë Ward 1, Sedona Sweeney 2, Lucy Platt 2, Matthew Hickman 1, Peter Vickerman 1 1 University of Bristol 2 London School of Hygiene and Tropical Medicine 1

06 October 2020 Disclosures and acknowledgements • • • Work funded by NIHR NKM and PV were supported by the National Institute for Drug Abuse. NKM was additionally funded by the University of California San Diego Center for AIDS Research (CFAR), a National Institute of Health (NIH) funded program. Lisa Maher is supported by an Australian National Health and Medical Research Council (NHMRC) Fellowship. PV and MH have received honoraria from Abbvie, MSD, Janssen and Gilead 2

06 October 2020 Background/Aims • Over 80% of new HCV infections in UK are in PWID • Needle and syringe programmes (NSP) are the main harm reduction strategy for blood borne viruses • No evidence of cost-effectiveness of NSP against HCV in UK • Investigate cost-effectiveness of current levels of high coverage needle and syringe provision (HCNSP - a clean needle for every injection) 3

Background • NIHR funded study into needle and syringe provision • • Pooled analysis of UK and Australian NSP data sets Systematic review of NSP and HCV data Costings of NSP at 3 UK settings Modelling of impact and cost-effectiveness of NSP at 3 UK settings Matt Hickman is presenting the systematic review results directly after this talk 4

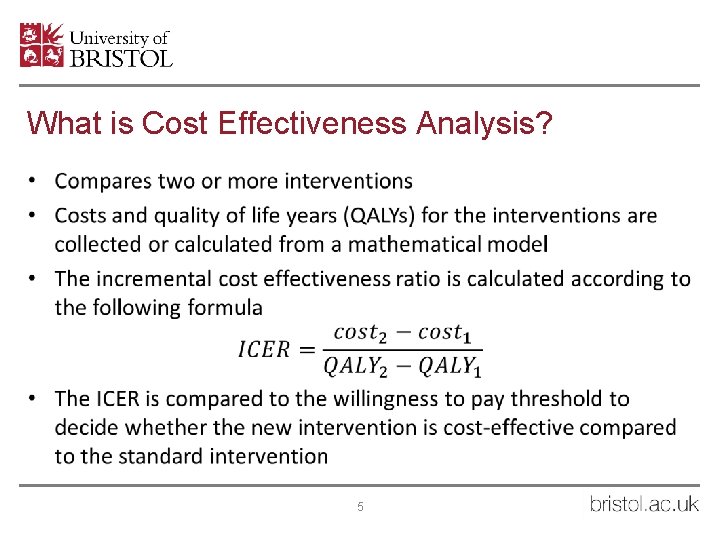
What is Cost Effectiveness Analysis? • 5

Methods • Stratified dynamic transmission model • Injecting duration • High Coverage Needle and Syringe Provision (HCNSP) and OST intervention • High/low injecting risk • Disease progression states • Follow ex-injectors in disease progression states 6

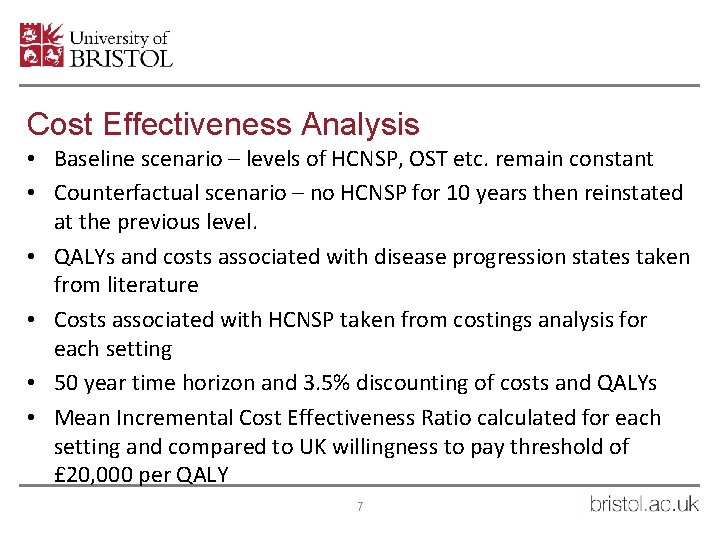
Cost Effectiveness Analysis • Baseline scenario – levels of HCNSP, OST etc. remain constant • Counterfactual scenario – no HCNSP for 10 years then reinstated at the previous level. • QALYs and costs associated with disease progression states taken from literature • Costs associated with HCNSP taken from costings analysis for each setting • 50 year time horizon and 3. 5% discounting of costs and QALYs • Mean Incremental Cost Effectiveness Ratio calculated for each setting and compared to UK willingness to pay threshold of £ 20, 000 per QALY 7

Parameterisation UAM survey for Bristol and Walsall Community surveys for Bristol 2004, 2006 and 2009 NESI survey for Dundee Population estimates from recent literature and local updates from collaborators • Odds ratios for HCNSP and OST effectiveness taken from the pooled analysis and systematic review • • Systematic Review and Pooled analysis carried out by Lucy Platt LSHTM 8

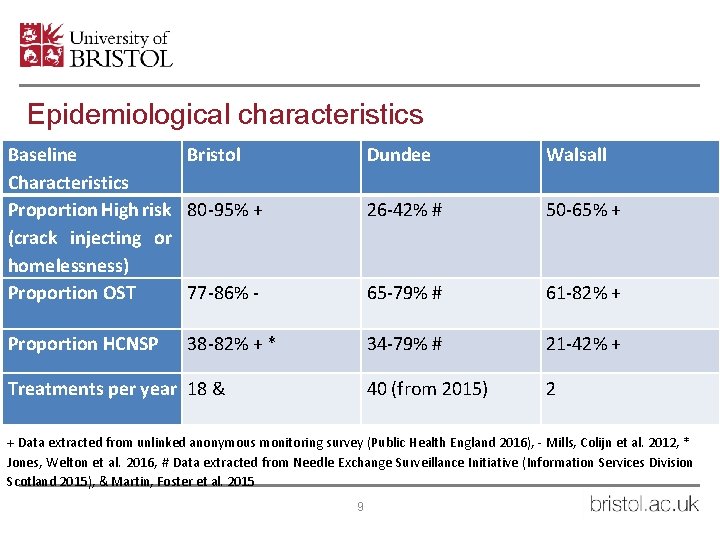
Epidemiological characteristics Baseline Bristol Characteristics Proportion High risk 80 -95% + (crack injecting or homelessness) Proportion OST 77 -86% - Dundee Walsall 26 -42% # 50 -65% + 65 -79% # 61 -82% + Proportion HCNSP 34 -79% # 21 -42% + 40 (from 2015) 2 38 -82% + * Treatments per year 18 & + Data extracted from unlinked anonymous monitoring survey (Public Health England 2016), - Mills, Colijn et al. 2012, * Jones, Welton et al. 2016, # Data extracted from Needle Exchange Surveillance Initiative (Information Services Division Scotland 2015), & Martin, Foster et al. 2015 9

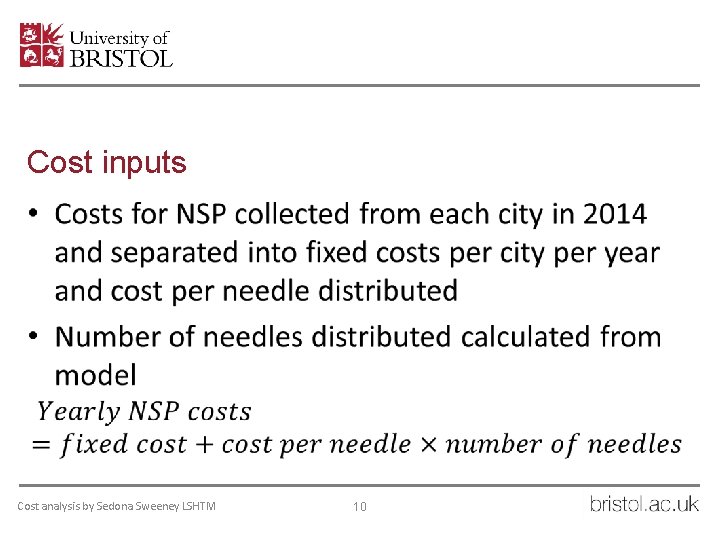
Cost inputs • Cost analysis by Sedona Sweeney LSHTM 10

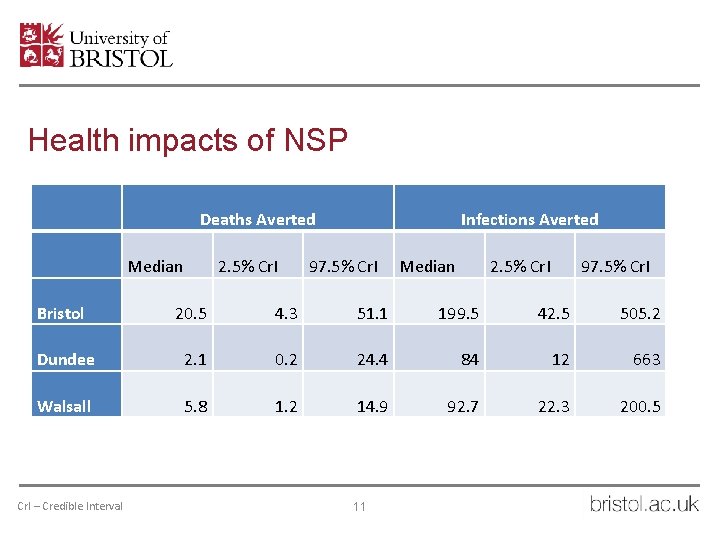
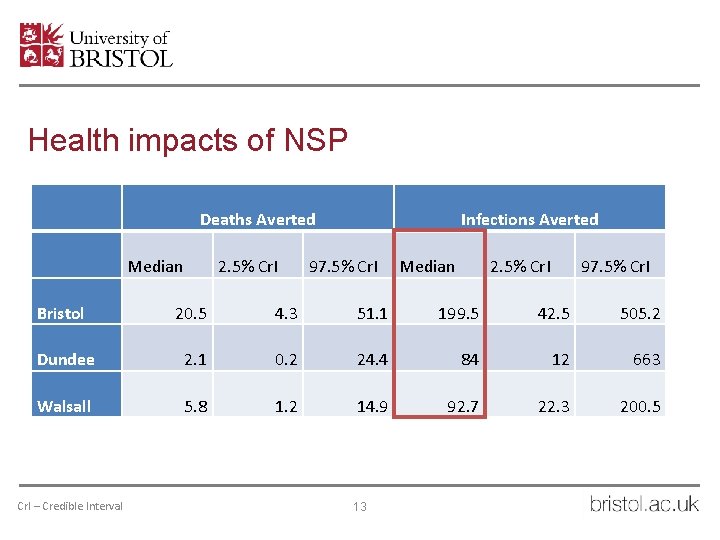
Health impacts of NSP Deaths Averted Median 2. 5% Cr. I Infections Averted 97. 5% Cr. I Median 2. 5% Cr. I 97. 5% Cr. I Bristol 20. 5 4. 3 51. 1 199. 5 42. 5 505. 2 Dundee 2. 1 0. 2 24. 4 84 12 663 Walsall 5. 8 1. 2 14. 9 92. 7 22. 3 200. 5 Cr. I – Credible Interval 11

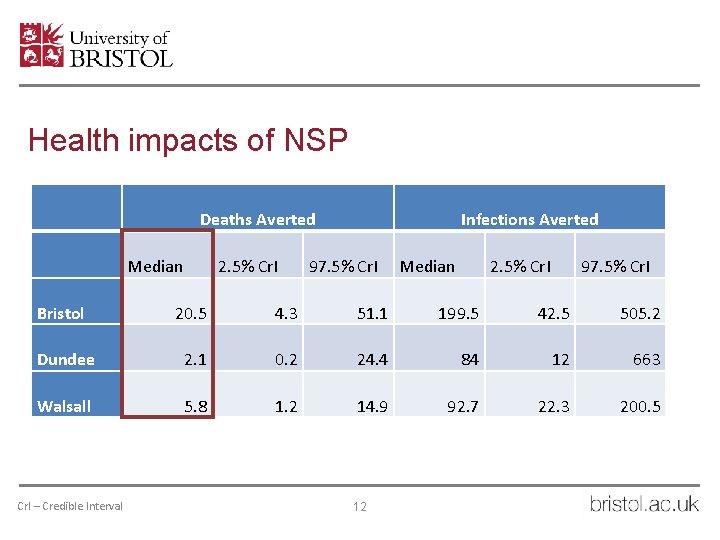
Health impacts of NSP Deaths Averted Median 2. 5% Cr. I Infections Averted 97. 5% Cr. I Median 2. 5% Cr. I 97. 5% Cr. I Bristol 20. 5 4. 3 51. 1 199. 5 42. 5 505. 2 Dundee 2. 1 0. 2 24. 4 84 12 663 Walsall 5. 8 1. 2 14. 9 92. 7 22. 3 200. 5 Cr. I – Credible Interval 12

Health impacts of NSP Deaths Averted Median 2. 5% Cr. I Infections Averted 97. 5% Cr. I Median 2. 5% Cr. I 97. 5% Cr. I Bristol 20. 5 4. 3 51. 1 199. 5 42. 5 505. 2 Dundee 2. 1 0. 2 24. 4 84 12 663 Walsall 5. 8 1. 2 14. 9 92. 7 22. 3 200. 5 Cr. I – Credible Interval 13

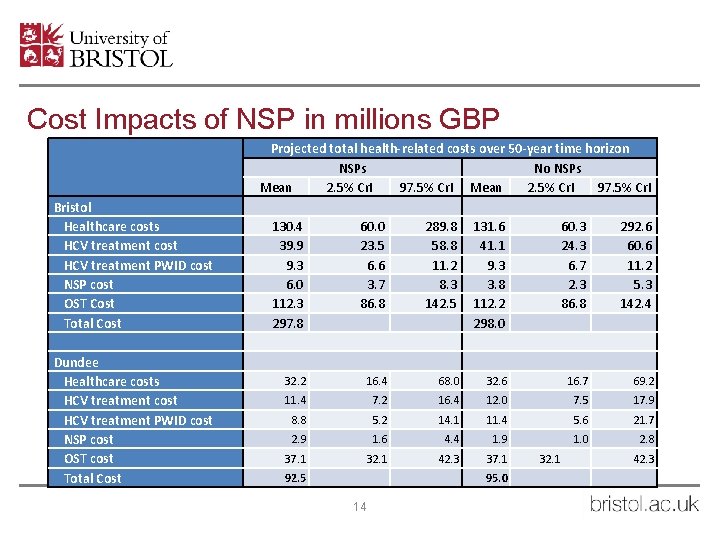
Cost Impacts of NSP in millions GBP Bristol Healthcare costs HCV treatment cost HCV treatment PWID cost NSP cost OST Cost Total Cost Dundee Healthcare costs HCV treatment cost HCV treatment PWID cost NSP cost OST cost Total Cost Projected total health-related costs over 50 -year time horizon NSPs No NSPs Mean 2. 5% Cr. I 97. 5% Cr. I 130. 4 39. 9 9. 3 6. 0 112. 3 297. 8 60. 0 23. 5 6. 6 3. 7 86. 8 289. 8 58. 8 11. 2 8. 3 142. 5 131. 6 41. 1 9. 3 3. 8 112. 2 298. 0 60. 3 24. 3 6. 7 2. 3 86. 8 292. 6 60. 6 11. 2 5. 3 142. 4 32. 2 16. 4 68. 0 32. 6 16. 7 69. 2 11. 4 7. 2 16. 4 12. 0 7. 5 17. 9 8. 8 5. 2 14. 1 11. 4 5. 6 21. 7 2. 9 1. 6 4. 4 1. 9 1. 0 2. 8 37. 1 32. 1 42. 3 37. 1 92. 5 95. 0 14 32. 1 42. 3

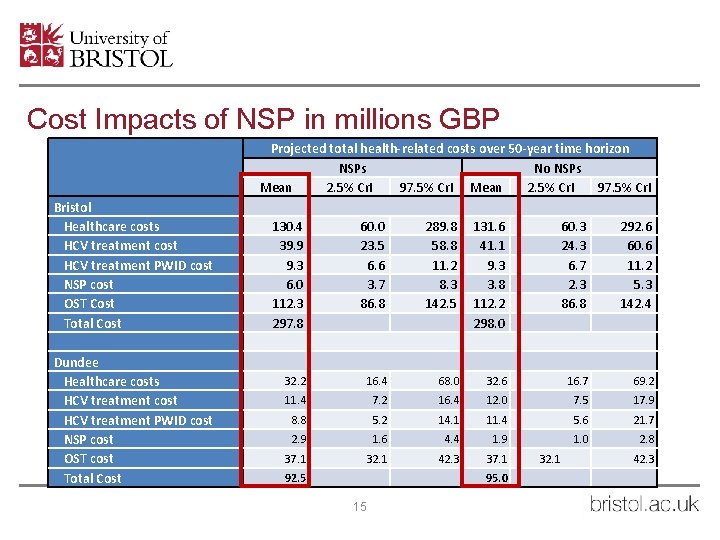
Cost Impacts of NSP in millions GBP Bristol Healthcare costs HCV treatment cost HCV treatment PWID cost NSP cost OST Cost Total Cost Dundee Healthcare costs HCV treatment cost HCV treatment PWID cost NSP cost OST cost Total Cost Projected total health-related costs over 50 -year time horizon NSPs No NSPs Mean 2. 5% Cr. I 97. 5% Cr. I 130. 4 39. 9 9. 3 6. 0 112. 3 297. 8 60. 0 23. 5 6. 6 3. 7 86. 8 289. 8 58. 8 11. 2 8. 3 142. 5 131. 6 41. 1 9. 3 3. 8 112. 2 298. 0 60. 3 24. 3 6. 7 2. 3 86. 8 292. 6 60. 6 11. 2 5. 3 142. 4 32. 2 16. 4 68. 0 32. 6 16. 7 69. 2 11. 4 7. 2 16. 4 12. 0 7. 5 17. 9 8. 8 5. 2 14. 1 11. 4 5. 6 21. 7 2. 9 1. 6 4. 4 1. 9 1. 0 2. 8 37. 1 32. 1 42. 3 37. 1 92. 5 95. 0 15 32. 1 42. 3

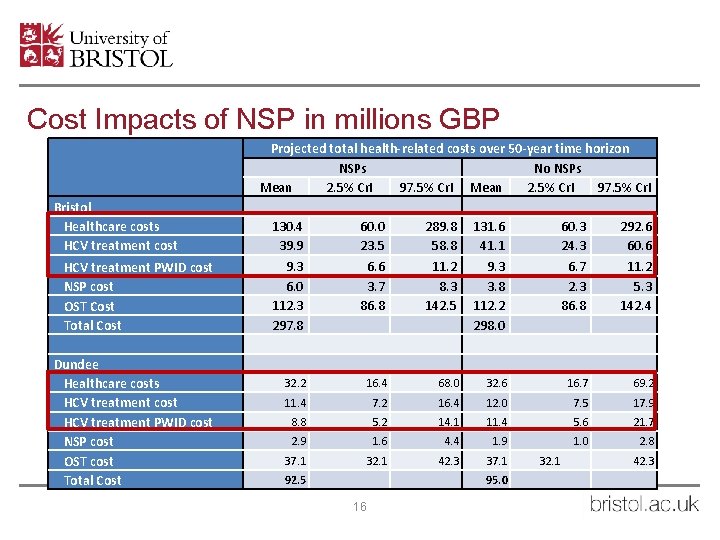
Cost Impacts of NSP in millions GBP Bristol Healthcare costs HCV treatment cost HCV treatment PWID cost NSP cost OST Cost Total Cost Dundee Healthcare costs HCV treatment cost HCV treatment PWID cost NSP cost OST cost Total Cost Projected total health-related costs over 50 -year time horizon NSPs No NSPs Mean 2. 5% Cr. I 97. 5% Cr. I 130. 4 39. 9 9. 3 6. 0 112. 3 297. 8 60. 0 23. 5 6. 6 3. 7 86. 8 289. 8 58. 8 11. 2 8. 3 142. 5 131. 6 41. 1 9. 3 3. 8 112. 2 298. 0 60. 3 24. 3 6. 7 2. 3 86. 8 292. 6 60. 6 11. 2 5. 3 142. 4 32. 2 16. 4 68. 0 32. 6 16. 7 69. 2 11. 4 7. 2 16. 4 12. 0 7. 5 17. 9 8. 8 5. 2 14. 1 11. 4 5. 6 21. 7 2. 9 1. 6 4. 4 1. 9 1. 0 2. 8 37. 1 32. 1 42. 3 37. 1 92. 5 95. 0 16 32. 1 42. 3

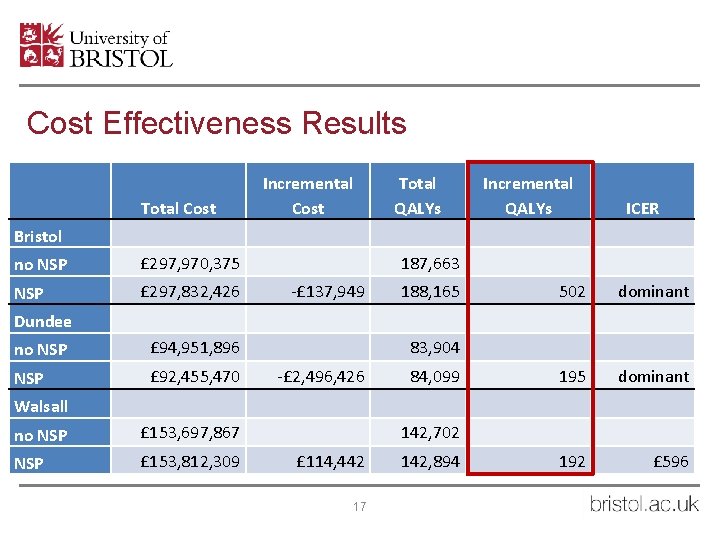
Cost Effectiveness Results Total Cost Incremental Cost Total QALYs Incremental QALYs ICER Bristol no NSP £ 297, 970, 375 NSP £ 297, 832, 426 187, 663 -£ 137, 949 188, 165 502 dominant 195 dominant 192 £ 596 Dundee no NSP £ 94, 951, 896 NSP £ 92, 455, 470 83, 904 -£ 2, 496, 426 84, 099 Walsall no NSP £ 153, 697, 867 NSP £ 153, 812, 309 142, 702 £ 114, 442 17 142, 894

Cost Effectiveness Results 18

Cost Effectiveness Results 19

Cost Effectiveness Results 20

Walsall and Dundee Results • Dundee also cost saving • Walsall cost-effective with mean ICER £ 594 per QALY • Results robust to sensitivity analysis on time horizon, discount rate, HCV treatment cost and undiagnosed healthcare cost 21

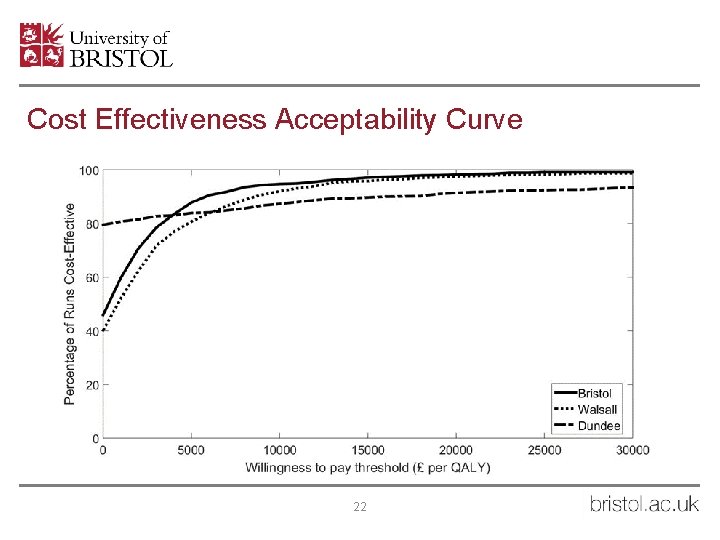
Cost Effectiveness Acceptability Curve 22

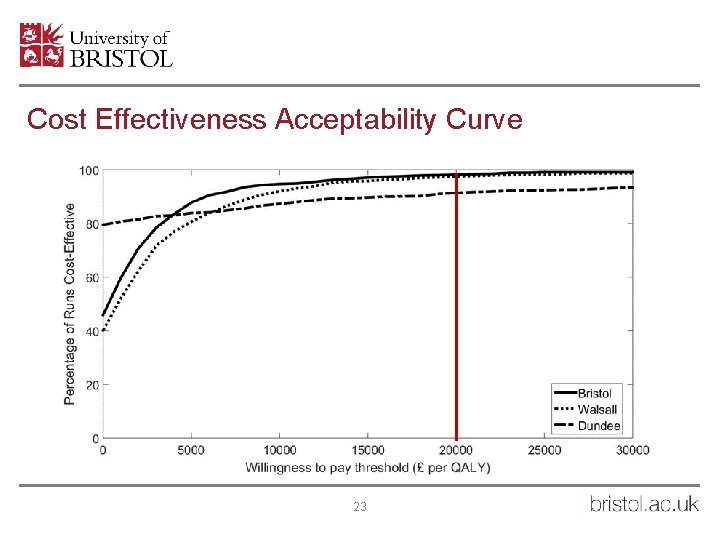
Cost Effectiveness Acceptability Curve 23

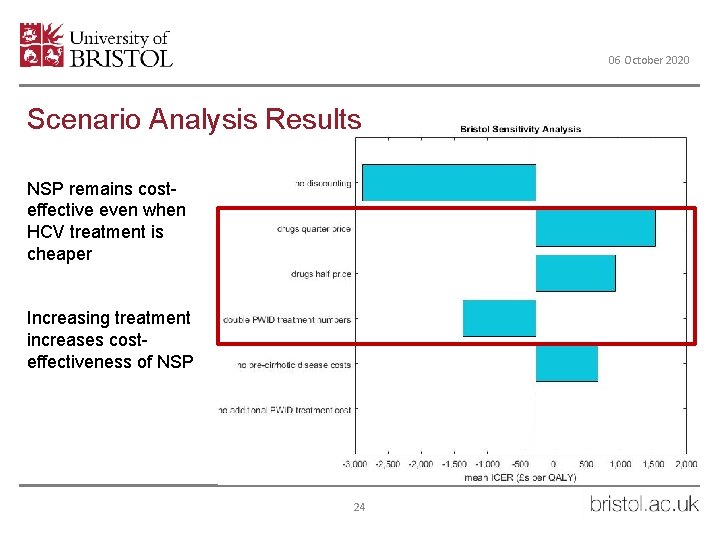
06 October 2020 Scenario Analysis Results NSP remains costeffective even when HCV treatment is cheaper Increasing treatment increases costeffectiveness of NSP 24

06 October 2020 Conclusions/implications • NSPs highly cost effective or cost saving in the UK • NSPs still cost effective when HCV treatment is cheaper • NSPs more cost effective when HCV treatment rate is increased • NSPs: Prevention is necessary alongside cure 25

Co-Author Acknowledgements • University of Bristol • Peter Vickerman • Matthew Hickman • LSHTM • Sedona Sweeney • Lucy Platt • Lorna Guinness • Lisa Maher (UNSW) • Vivian Hope (LJMU) • Josie Smith (PHW) • Rachel Ayres (Bristol Drugs Project) • Ingrid Hainey (Dundee Cairn Centre) • Tracy Chamberlin (Addaction Walsall) 26

References • • Information Services Division Scotland (2015). Injecting equipment provision in Scotland survey 2013/14. Scotland. Jones, H. E. , N. J. Welton, A. Ades, M. Pierce, W. Davies, B. Coleman, T. Millar and M. Hickman (2016). "Problem drug use prevalence estimation revisited: heterogeneity in capture–recapture and the role of external evidence. " Martin, N. K. , G. R. Foster, J. Vilar, S. Ryder, M. E. Cramp, F. Gordon, J. F. Dillon, N. Craine, H. Busse, A. Clements, S. J. Hutchinson, A. Ustianowski, M. Ramsay, D. J. Goldberg, W. Irving, V. Hope, D. De Angelis, M. Lyons, P. Vickerman and M. Hickman (2015). "HCV treatment rates and sustained viral response among people who inject drugs in seven UK sites: real world results and modelling of treatment impact. " Journal of Viral Hepatitis 22(4): 399 -408. Mills, H. L. , C. Colijn, P. Vickerman, D. Leslie, V. Hope and M. Hickman (2012). "Respondent driven sampling and community structure in a population of injecting drug users, Bristol, UK. " Drug and Alcohol Dependence 126(3): 324 -332. Public Health England (2016). People who inject drugs: HIV and viral hepatitis unlinked anonymous monitoring survey tables (pyschoactive): 2016 update. London. Sweeting, M. J. , et al. (2007). "The burden of hepatitis C in England. " Journal of Viral Hepatitis 14(8): 570 -576. Micallef, J. M. , et al. (2006). "Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. " J Viral Hepat 13(1): 34 -41. 27
 Intradermal injection procedure
Intradermal injection procedure The provision of a public good generates a
The provision of a public good generates a Read syringe
Read syringe 300 mcg on insulin syringe
300 mcg on insulin syringe Halo vial converter
Halo vial converter Disposable syringe discovered
Disposable syringe discovered Tresiba moa
Tresiba moa Normal values abg
Normal values abg Glabella botox
Glabella botox Myomics
Myomics Rumus obat injeksi
Rumus obat injeksi Syringe sizes chart
Syringe sizes chart Movie syringe
Movie syringe Low dead space syringe
Low dead space syringe Dlco test
Dlco test Industrial catering
Industrial catering The provision and use of work equipment
The provision and use of work equipment Bad debts and provision for bad debts
Bad debts and provision for bad debts Costed provision map example
Costed provision map example Samba tool domain provision
Samba tool domain provision Summarise types of early years provision
Summarise types of early years provision Core service provision
Core service provision Servicios ecosistémicos de provisión
Servicios ecosistémicos de provisión Provision of unrealised profit
Provision of unrealised profit Qué acto aseguro nuestra salvación
Qué acto aseguro nuestra salvación Irrecoverable debts account
Irrecoverable debts account Individual tax computation format
Individual tax computation format Accounting for hire purchase
Accounting for hire purchase