Technology Title Oneline Description Name Ph D Title









- Slides: 12

Technology Title One-line Description Name, Ph. D Title Department Rutgers University Name 2, Ph. D; Title Name 3, Ph. D; Title 8/29/2019 Confidential 1

Introduction • “Elevator pitch” – a simple mission statement, no more than 2 -3 sentences, that encapsulates: o What your technology is and how it works, in “plain English”; o What specific problem it solves; o Why your technology is unique and superior; and o Who actually uses your product (if appropriate). • A picture or diagram of your technology, if appropriate. • This slide should be crafted for a generalist and non-scientist. 8/29/2019 Confidential 2

Problem & Market Need • What is the unmet market need? What is the problem you are trying to solve? • What is the estimated market size specific to your technology? 8/29/2019 Confidential 3

The Innovation - Summary • Visual display of your product/service. o What does it do? o How does it solve the market need? • Compare and contrast to current solutions/gold standard. o How is your solution unique? o What are the advantages? o What are the risks and dis-advantages? 8/29/2019 Confidential 4

The Innovation - Details Part 1 • Describe one specific aspect or improvement that your technology offers (i. e. , one aspect per slide). • Include supporting data, evidence, charts, as appropriate. 8/29/2019 Confidential 5

The Innovation - Details Part 2 (3 additional slides, max) • Describe a specific aspect / improvement that your technology offers. • Include supporting data, evidence, charts, as appropriate. 8/29/2019 Confidential 6

Current Stage of Development • What is the IP/patent status? o Other than Rutgers, what other institution(s), if any, co-own(s) the technology? • What is the current stage of the technology? i. e. , “Where are you today? ” o E. g. , “We achieved …” or “We discovered that …” o E. g. , “Our lead compounds exhibit …” o E. g. , “We have a working prototype that does. . . ” o E. g. , “We are ready to perform …” o E. g. , “We identified partners to help manufacture components of …” o This section should include a combination of the above, and serves as a segue to the next slide. 8/29/2019 Confidential 7

Goals & Milestones – (See Next Slide for an example) • Near-term goals (next steps to validate commerciality) • Experiments, MVP (minimum viable product) • Customer discovery exercises • Gaps to be addressed with current round of funding: o What will this funding help you achieve? o What meaningful results do you expect to generate? o What aspect of the technology are you “de-risking” (e. g. , toxicity, efficacy, etc. ) • Multi-year timeline of product development to commercialization. o E. g. , pre-clinical, product launch. o What key research needs to be done at each stage? o What specifically are you “de-risking” at each stage? • How much (further) funding is likely required at each stage. (Optional) o This sub-section would be helpful if the tech requires materially different funding or timeline of development compared to “standard” regulatory processes. • Are there other broader potential fields of use or commercialization aspects for this technology? 8/29/2019 Confidential 8

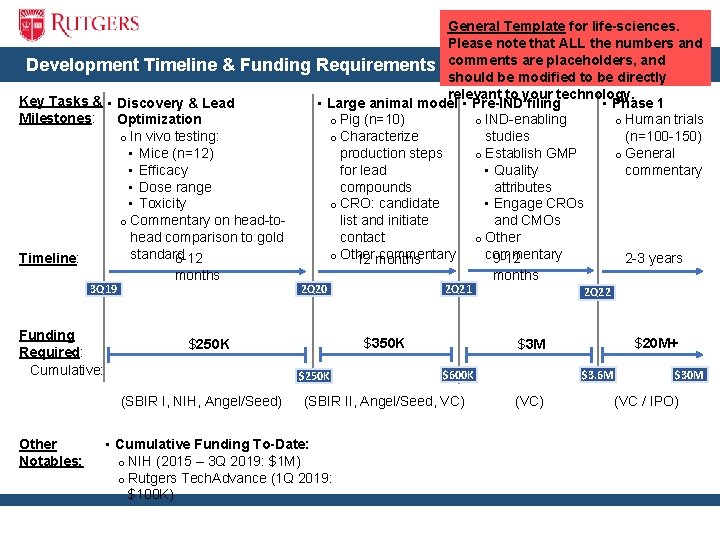
Development Timeline & Funding Key Tasks & • Discovery & Lead Milestones: Optimization o In vivo testing: • Mice (n=12) • Efficacy • Dose range • Toxicity o Commentary on head-tohead comparison to gold standard Timeline: 6 -12 3 Q 19 Funding Required: Cumulative: months 2 Q 20 2 Q 21 $350 K $250 K (SBIR I, NIH, Angel/Seed) Other Notables: General Template for life-sciences. Please note that ALL the numbers and Requirements comments are placeholders, and should be modified to be directly relevant to your technology. • Phase 1 • Large animal model • Pre-IND filing o Human trials o Pig (n=10) o IND-enabling (n=100 -150) o Characterize studies o General production steps o Establish GMP commentary for lead • Quality compounds attributes o CRO: candidate • Engage CROs list and initiate and CMOs contact o Other commentary 9 -12 2 -3 years 12 months 2 Q 22 $20 M+ $3 M $600 K (SBIR II, Angel/Seed, VC) • Cumulative Funding To-Date: o NIH (2015 – 3 Q 2019: $1 M) o Rutgers Tech. Advance (1 Q 2019: $100 K) months $3. 6 M (VC) $30 M (VC / IPO)

The Team • Include pictures of team members, if appropriate. • If possible, highlight skillsets and knowledge base that are complementary. • An ideal team would have: Scientific expertise, Clinical expertise, Commercialization expertise. • Include a “Commercialization Track Record”, if any, as a separate slide: o A summary highlight of products that your team was directly involved with, that are currently (1) commercial, (2) in development, and/or (3) in clinical studies or use. • If you have a truly unique team and track record (successful commercialization, seasoned business founders, expert clinicians, etc. ), this slide could be the first or second slide of the presentation. 8/29/2019 Confidential 10

THANK YOU 8/29/2019 Confidential 11

Appendix (multiple slides as appropriate) • More data and evidence supporting your technology. • More details on market size, segments of the market, and opportunity. 8/29/2019 Confidential 12