Target 2010 The Toyota of Pharma Tom van













- Slides: 30

Target 2010 The Toyota of Pharma Tom van Laar Head of Global Technical Operations Novartis Pharma AG © Novartis Pharma AG

Agenda 1. Novartis Pharma Technical Operations Introduction 2. Novartis Tech. Ops “Toyota of Pharma” Vision 3. Risk Management Strategies and Lessons Learned 4. Q & A 2 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

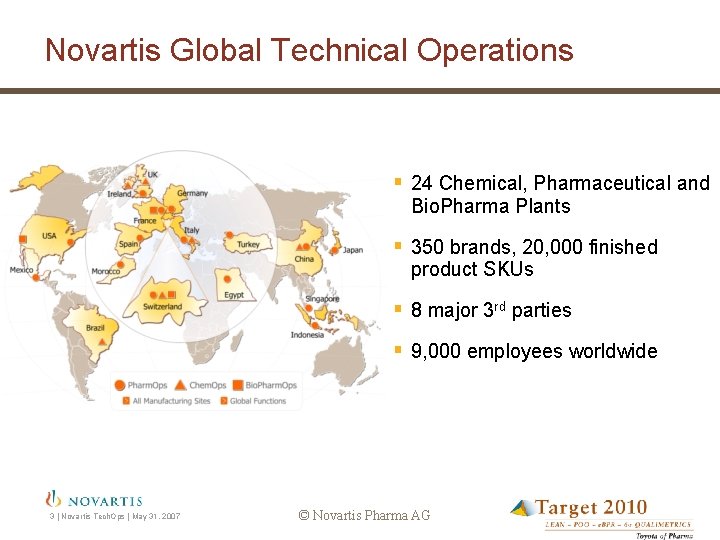
Novartis Global Technical Operations § 24 Chemical, Pharmaceutical and Bio. Pharma Plants § 350 brands, 20, 000 finished product SKUs § 8 major 3 rd parties § 9, 000 employees worldwide 3 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

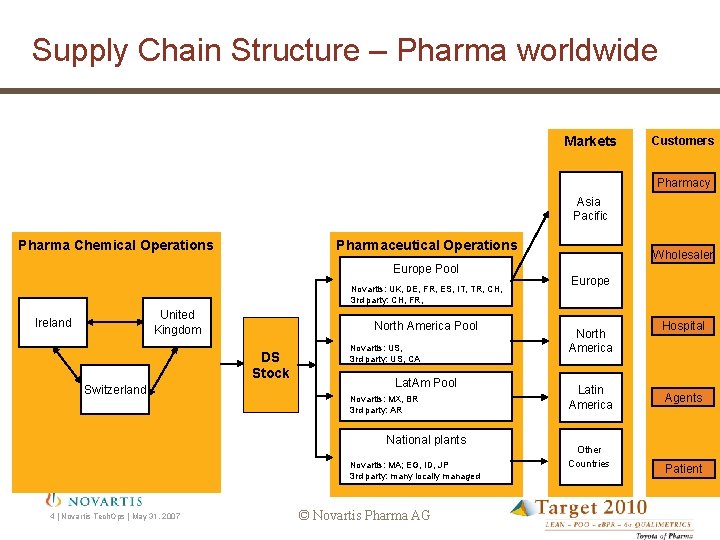
Supply Chain Structure – Pharma worldwide Markets Customers Pharmacy Asia Pacific Pharma Chemical Operations Pharmaceutical Operations Europe Pool Novartis: UK, DE, FR, ES, IT, TR, CH, 3 rd party: CH, FR, United Kingdom Ireland North America Pool DS Stock Switzerland Novartis: US, 3 rd party: US, CA Lat. Am Pool Novartis: MX, BR 3 rd party: AR National plants Novartis: MA; EG, ID, JP 3 rd party: many locally managed 4 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG Wholesaler Europe North America Latin America Other Countries Hospital Agents Patient

Agenda 1. Novartis Pharma Technical Operations Introduction 2. Novartis Tech. Ops “Toyota of Pharma” Vision 3. Risk Management Strategies and Lessons Learned 4. Q & A 5 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

Key Challenges Facing Big Pharma Opportunities Challenges § § Positive demographic change Unmet medical needs Growth in biologics Growth in emerging markets § Time to market § Pricing pressure, generic competition, shortening patent lives § Increased FDA/EMEA scrutiny § Increased role of non-physician stakeholders / channel change (direct distribution) 6 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

Development of Tech. Ops Vision / Target 2010 § Why Change? • Novartis Tech. Ops already strong in 2005 (low costs, high quality, etc. ) relative to other Pharma companies. § Prepare for the future, 5+ years from a position of strength. § Learn from other industries, e. g. Toyota. 7 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

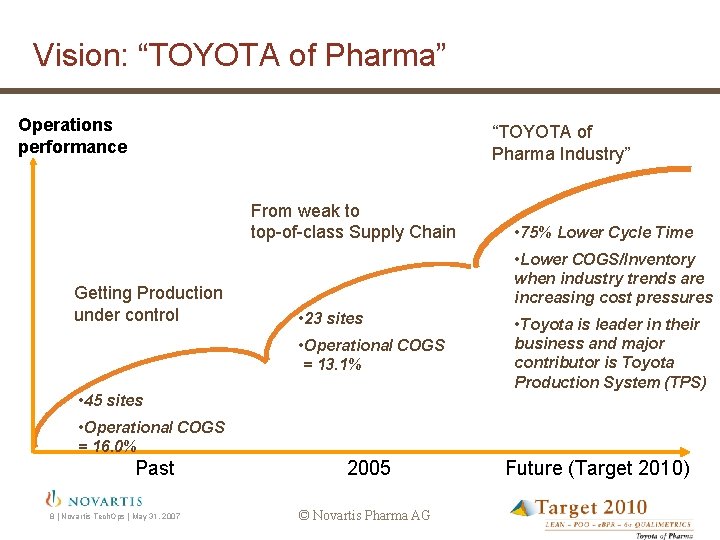
Vision: “TOYOTA of Pharma” Operations performance “TOYOTA of Pharma Industry” From weak to top-of-class Supply Chain Getting Production under control • 75% Lower Cycle Time • Lower COGS/Inventory when industry trends are increasing cost pressures • 23 sites • Operational COGS = 13. 1% • 45 sites • Toyota is leader in their business and major contributor is Toyota Production System (TPS) • Operational COGS = 16. 0% Past 8 | Novartis Tech. Ops | May 31, 2007 2005 © Novartis Pharma AG Future (Target 2010)

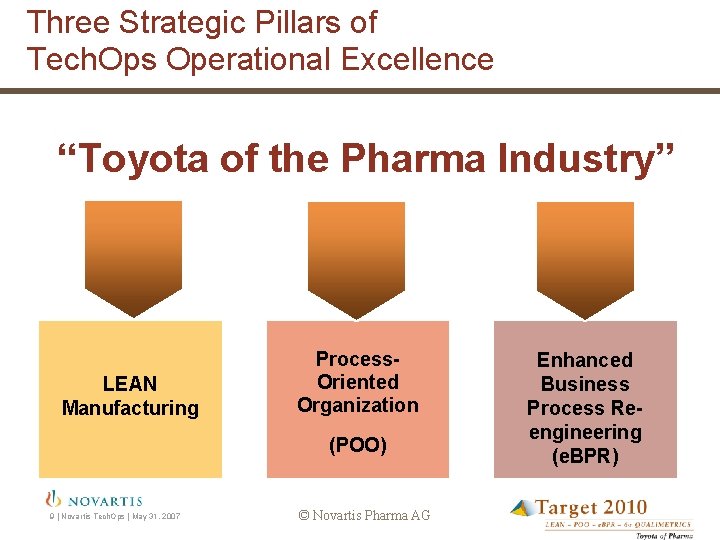
Three Strategic Pillars of Tech. Ops Operational Excellence “Toyota of the Pharma Industry” LEAN Manufacturing Process. Oriented Organization (POO) 9 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG Enhanced Business Process Reengineering (e. BPR)

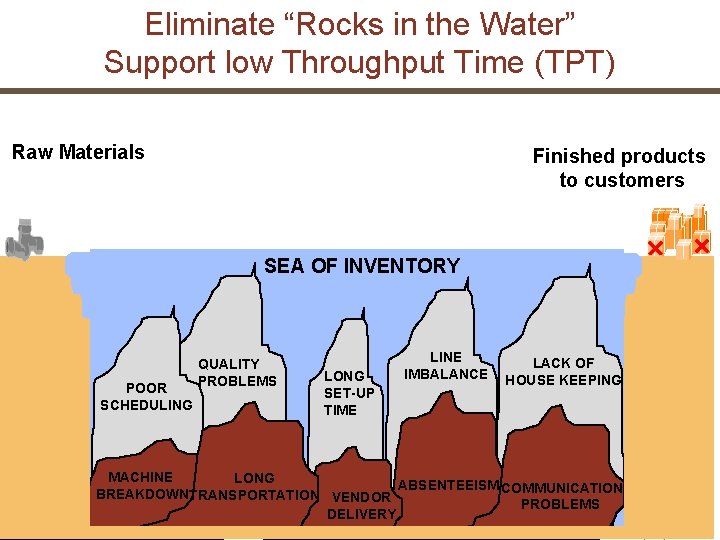
Pillar 1: LEAN Manufacturing § Synchronisation of manufacturing • Driven by demand rather than forecast • Continuous flow of work and people • Logical rhythm through the supply chain • Moving forward via downstream signals § Elimination of waste • Non-value adding steps removed • Value adding steps broken down and linked • Problems solved at root cause (eliminate “rocks in water”) 10 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

Eliminate “Rocks in the Water” Support low Throughput Time (TPT) Raw Materials Finished products to customers SEA OF INVENTORY POOR SCHEDULING QUALITY PROBLEMS LONG SET-UP TIME LINE IMBALANCE LACK OF HOUSE KEEPING MACHINE LONG ABSENTEEISM COMMUNICATION BREAKDOWNTRANSPORTATION VENDOR PROBLEMS 11 | Novartis Tech. Ops | May 31, 2007 DELIVERY © Novartis Pharma AG

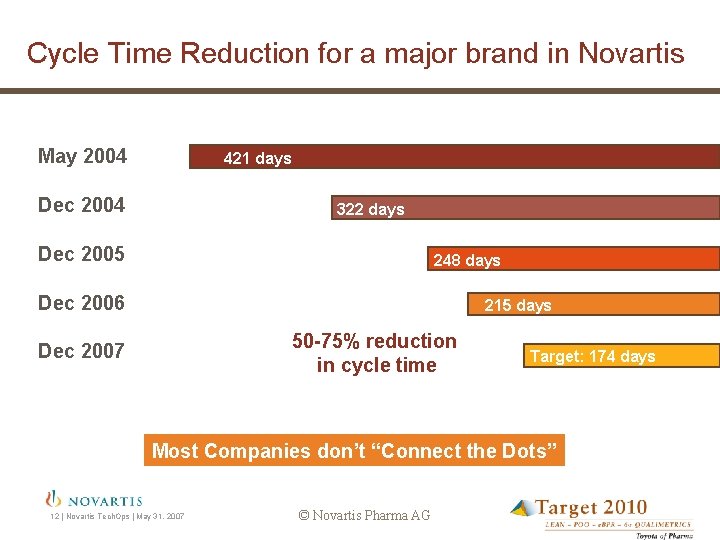
Cycle Time Reduction for a major brand in Novartis May 2004 421 days Dec 2004 322 days Dec 2005 248 days Dec 2006 215 days 50 -75% reduction in cycle time Dec 2007 Target: 174 days Most Companies don’t “Connect the Dots” 12 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

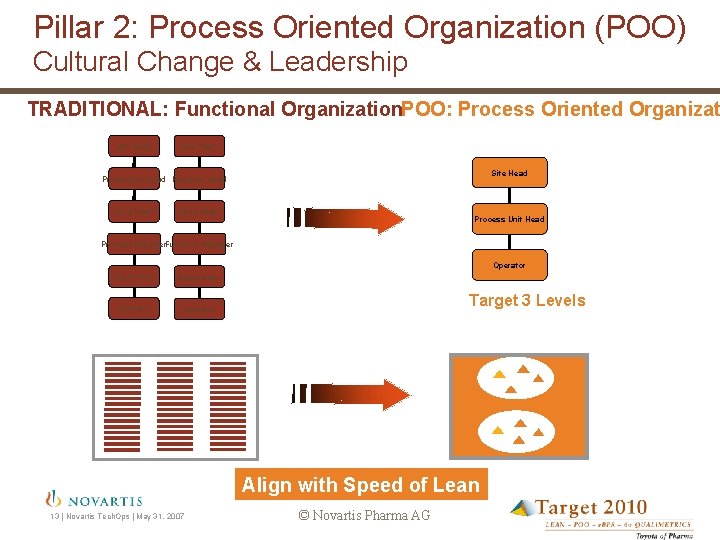
Pillar 2: Process Oriented Organization (POO) Cultural Change & Leadership TRADITIONAL: Functional Organization. POO: Process Oriented Organizat Site Head Production Head Function Head Area Head Process Unit Head Process Manager. Function Manager Operator Supervisor Operator Target 3 Levels Align with Speed of Lean 13 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

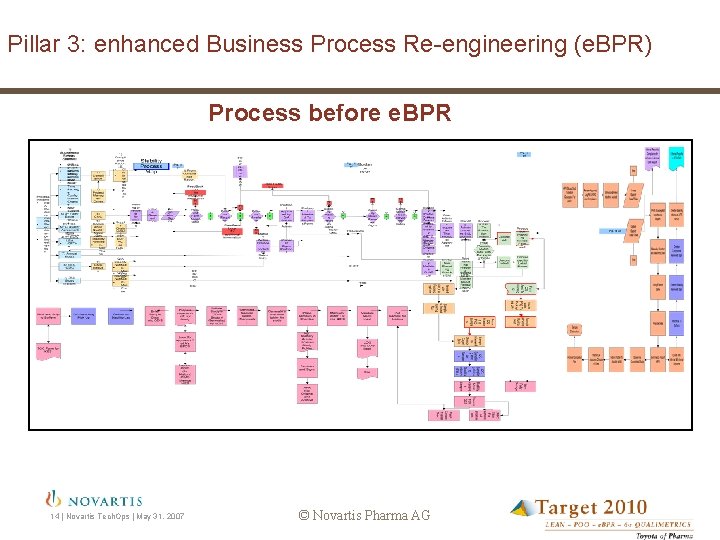
Pillar 3: enhanced Business Process Re-engineering (e. BPR) Process before e. BPR 14 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

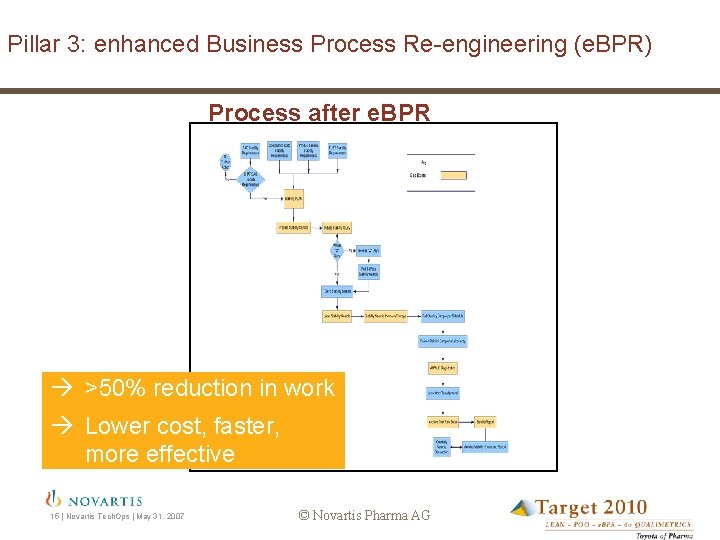
Pillar 3: enhanced Business Process Re-engineering (e. BPR) Process after e. BPR >50% reduction in work Lower cost, faster, more effective 15 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

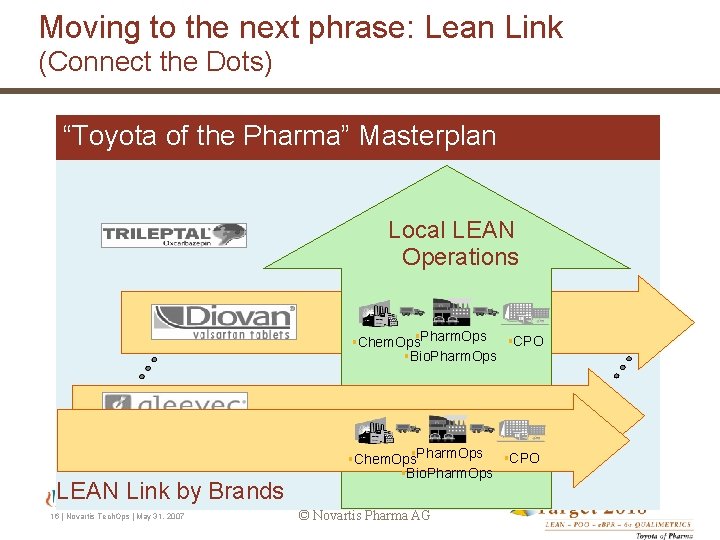
Moving to the next phrase: Lean Link (Connect the Dots) “Toyota of the Pharma” Masterplan Local LEAN Operations §CPO §Chem. Ops§Pharm. Ops §Bio. Pharm. Ops LEAN Link by Brands 16 | Novartis Tech. Ops | May 31, 2007 §CPO §Chem. Ops§Pharm. Ops §Bio. Pharm. Ops © Novartis Pharma AG

Key Lean/POO/e. BPR Learning Points 1. Blue Sky / Practical Vision Techniques 2. Definition of “waste”. Re-engineering of ANY work process to eliminate waste (assume 50%). Toyota aims for 80% value added time. 3. Keep it simple! (sometimes just let the new team “figure out how to do it”) 4. Eliminate unnecessary levels and functions (force empowerment & crossfunctional behaviors) 5. Aligning 3 strategic pillars: fix/simplify/re-engineer processes (LEAN/e. BPR) in addition to changing culture and organization (POO) 17 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

Agenda 1. Novartis Pharma Technical Operations Introduction 2. Novartis Tech. Ops “Toyota of Pharma” Vision 3. Risk Management Strategies and Lessons Learned 4. Q & A 18 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

Pharma Supply Chain Environment § Heavily regulated with strict operating guidelines § Improving supply speed & reliability in addition to reducing cost § Improving availability in addition to reducing inventory § Complex global supply chain networks & long supply chain cycle time (typically > 1 year) § Traceability and counterfeits § Balancing valuable capacity allocation for mature products with new product launches 19 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

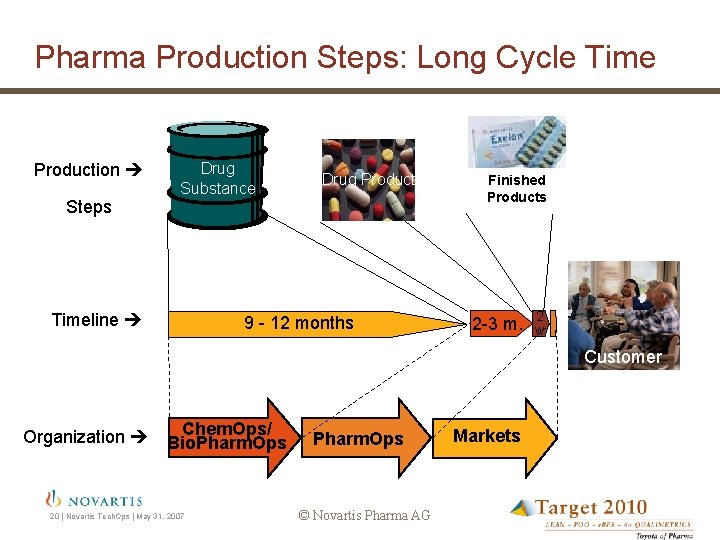
Pharma Production Steps: Long Cycle Time Production Steps Drug Substance Timeline Drug Product 9 - 12 months Finished Products 2 -3 m. 2 w Customer Organization Chem. Ops/ Bio. Pharm. Ops 20 | Novartis Tech. Ops | May 31, 2007 Pharm. Ops © Novartis Pharma AG Markets

Pharma Supply Chain Trends § “Leaner” supply chains to reduce costs and improve flexibility § Increased manufacturing in low cost countries § Accelerated product development time means that manufacturing could be locked in at an earlier point in the product life cycle § New technologies: processes, biological, IT § Devices and service items for new products § Wholesalers adopt a forward integration strategy to own pharmacy chains § Direct distribution to patients & pharmacies 21 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

Major Risk 1: 100% supply of life saving drugs and high margin brands Supply comes 1 st before low inventory level § Different from many other industries. E. g. People can probably wait for a new PC model, but not a life saving drug. § Availability of top brands affect profitability due to high gross margins and relatively low COGS in Pharma industry, esp. the substitution in high value markets. § Revenue from major blockbuster drugs financially feed the R&D engine & drive further innovation 22 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

Major Risk 2: Quality & Compliance Strategies § From fire-fighting to proactive mindsets § Play a key role in eliminating “rocks in water” § Process Analytical Technology (PAT) align with Lean to improve process capabilities with real time release(build in Quality-Quality by Design) 23 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

Major Risk 3: Speed to Market; Responsive to Market Changes Time: speed to market New launches uncertainties & risks § FDA approval timing and dosages § Variable demand forecasting § Complexity sourcing with devices and biologics § Technical manufacturing process Quality Cost Planned launches in Novartis in 2007 24 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

Building speed-to-market capabilities to support new launches § Cross functional teams (with Marketing, sales, development, regulatory, BD&L departments) create and implement global and integrated launch plans § Comprehensive risk & sensitivity analysis for up/down side scenarios from both drug demand supply § Building robust and responsive & flexible supply during and after product launch (Lean / POO / e. BPR / 6 Sigma) TPT > 300 Days => Can’t Respond Fast 25 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

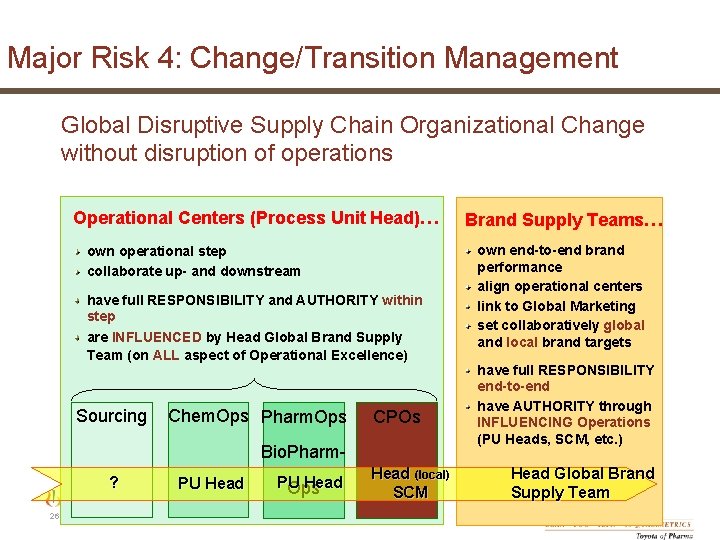
Major Risk 4: Change/Transition Management Global Disruptive Supply Chain Organizational Change without disruption of operations Operational Centers (Process Unit Head)… own operational step collaborate up- and downstream have full RESPONSIBILITY and AUTHORITY within step are INFLUENCED by Head Global Brand Supply Team (on ALL aspect of Operational Excellence) Sourcing Chem. Ops Pharm. Ops CPOs Bio. Pharm? PU Head 26 | Novartis Tech. Ops | May 31, 2007 PU Head Ops Head (local) SCM © Novartis Pharma AG Brand Supply Teams… own end-to-end brand performance align operational centers link to Global Marketing set collaboratively global and local brand targets have full RESPONSIBILITY end-to-end have AUTHORITY through INFLUENCING Operations (PU Heads, SCM, etc. ) Head Global Brand Supply Team

Key Change Management Learning Points 1. Fundamental Change but well planned to minimize risks 2. Get risk averse senior leadership on board. Senior management’s commitment and focus. 3. Selecting right people in key positions, such as site heads, PU heads, Site HR Heads, as change agents to drive the change 4. Pushing change & obtaining “buy-in”. Moving fast but also providing training and incentives and give people time to come on board. 5. Team building and leadership training 6. People tend to limit what they think needs Changing & Say “We’ve Already Fixed That” and they are almost always wrong. 27 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

Major Risk 5: Manufacturing Investment Risks and Strategies Risks Strategies § It takes 2 -5 years & hundreds of § When to build is a key factor for risk millions of $s to bring on new capacity § Highly variable demand mitigation § “Time, cost, control and flexibility” (Lean) forecasting § Supply chain and manufacturing processes for untested drug must be put into place while managing the risk of a drug’s failure to receive FDA approval § Key factors: company portfolio, pipeline, volume growth, compliance, profitability, labor skills and cost, equipment (dedicated vs. multipurpose), IP, etc. § Wrong investment decisions • Plants idle when the drug fails to receive FDA approval • Stock out, delayed launches 28 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

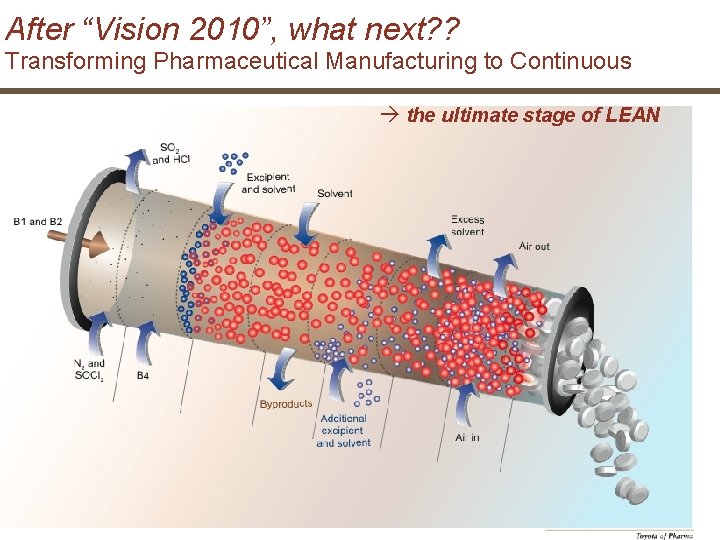
After “Vision 2010”, what next? ? Transforming Pharmaceutical Manufacturing to Continuous the ultimate stage of LEAN 29 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG

Q&A 30 | Novartis Tech. Ops | May 31, 2007 © Novartis Pharma AG
 Toyota pharma
Toyota pharma Primary target market and secondary target market
Primary target market and secondary target market Go 910
Go 910 What does tom symbolize in the devil and tom walker
What does tom symbolize in the devil and tom walker Cơ vân cắt dọc
Cơ vân cắt dọc Pharma plus software
Pharma plus software Pharmaceutical science atar
Pharmaceutical science atar Indian pharma association
Indian pharma association 97890 mobile number
97890 mobile number Helvoet pharma alken
Helvoet pharma alken Pharmaceutical due diligence checklist
Pharmaceutical due diligence checklist Pharma test dissolution apparatus
Pharma test dissolution apparatus Denk pharma wikipedia
Denk pharma wikipedia Orion pharma animal health
Orion pharma animal health Pdcbenefits
Pdcbenefits Lex group bulgaria
Lex group bulgaria Ego pharma birthday 10th february 2006
Ego pharma birthday 10th february 2006 Denk farma
Denk farma Ehci pharma training
Ehci pharma training Chem pharma impex
Chem pharma impex Water for injection process diagram
Water for injection process diagram Healthy pharma
Healthy pharma Catalyst pharma consulting
Catalyst pharma consulting Pics scheme
Pics scheme Opc pharma
Opc pharma Isis pharma costa rica
Isis pharma costa rica Bimal pharma pvt ltd
Bimal pharma pvt ltd Veyx la
Veyx la Eric percher
Eric percher Pharma sim
Pharma sim Valeant pharmaceuticals accounting scandal
Valeant pharmaceuticals accounting scandal




















































