T Take Notes I Interact with your notes






- Slides: 21

T = Take Notes I = Interact with your notes P = Practice with plenty of repetition S = Self-test


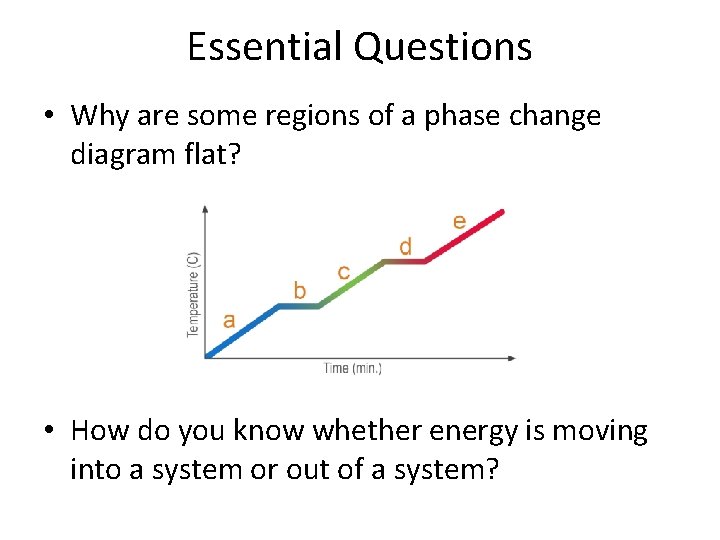
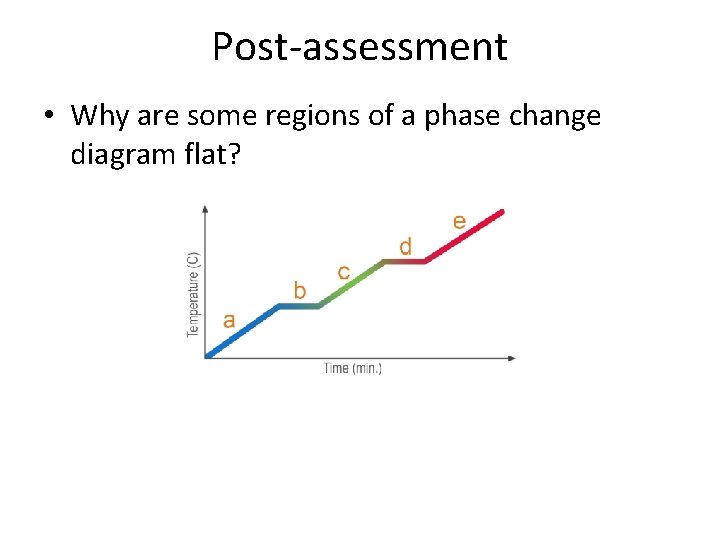
Essential Questions • Why are some regions of a phase change diagram flat? • How do you know whether energy is moving into a system or out of a system?

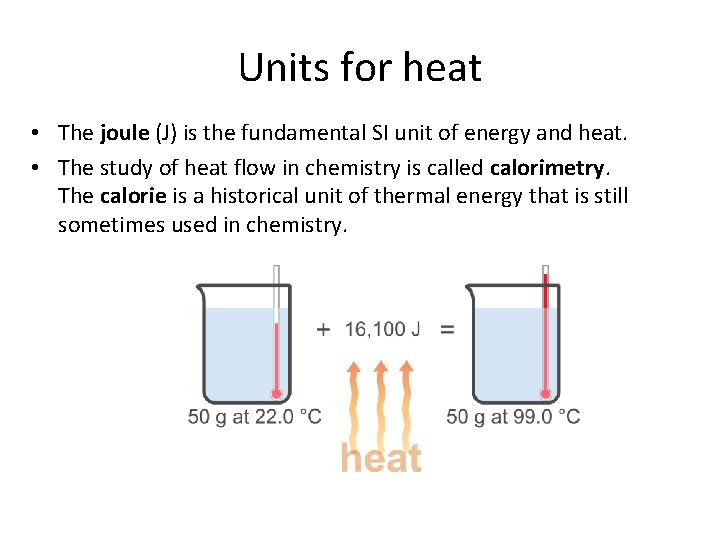
Units for heat • The joule (J) is the fundamental SI unit of energy and heat. • The study of heat flow in chemistry is called calorimetry. The calorie is a historical unit of thermal energy that is still sometimes used in chemistry.

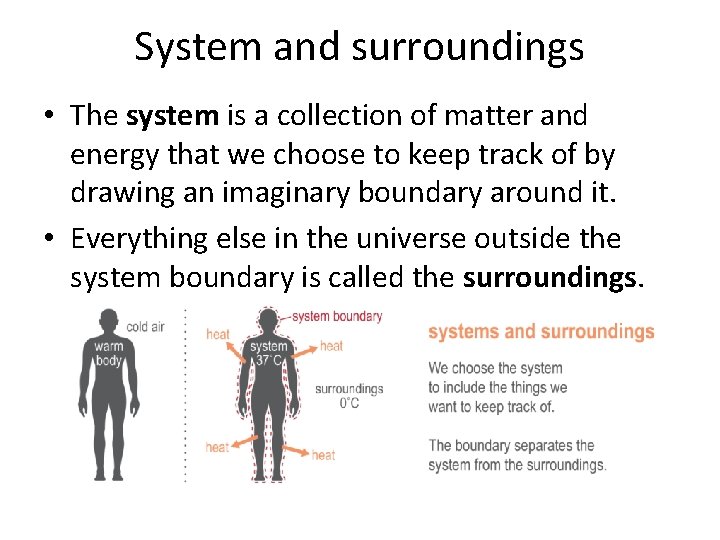
System and surroundings • The system is a collection of matter and energy that we choose to keep track of by drawing an imaginary boundary around it. • Everything else in the universe outside the system boundary is called the surroundings.

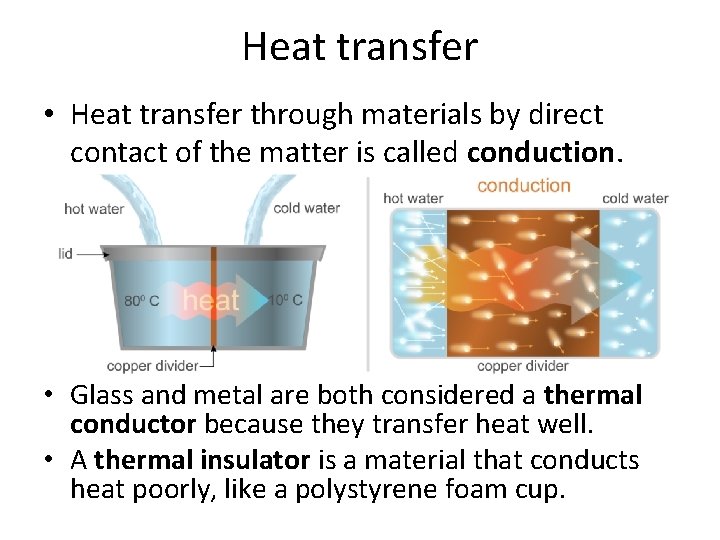
Heat transfer • Heat transfer through materials by direct contact of the matter is called conduction. • Glass and metal are both considered a thermal conductor because they transfer heat well. • A thermal insulator is a material that conducts heat poorly, like a polystyrene foam cup.

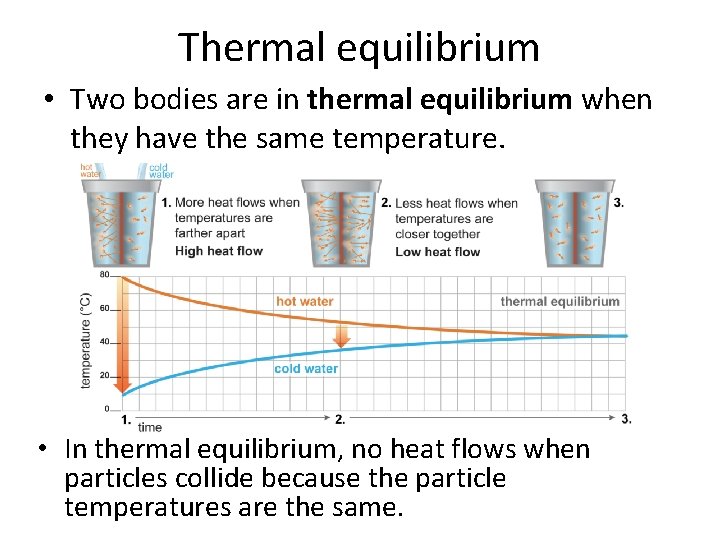
Thermal equilibrium • Two bodies are in thermal equilibrium when they have the same temperature. • In thermal equilibrium, no heat flows when particles collide because the particle temperatures are the same.

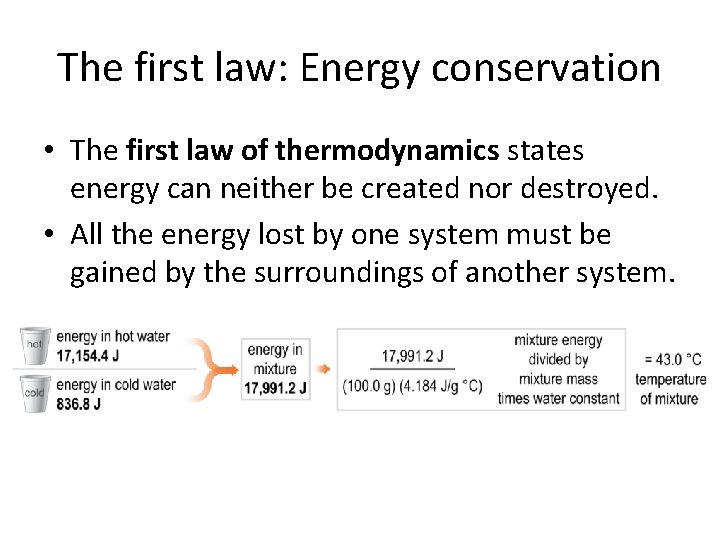
The first law: Energy conservation • The first law of thermodynamics states energy can neither be created nor destroyed. • All the energy lost by one system must be gained by the surroundings of another system.

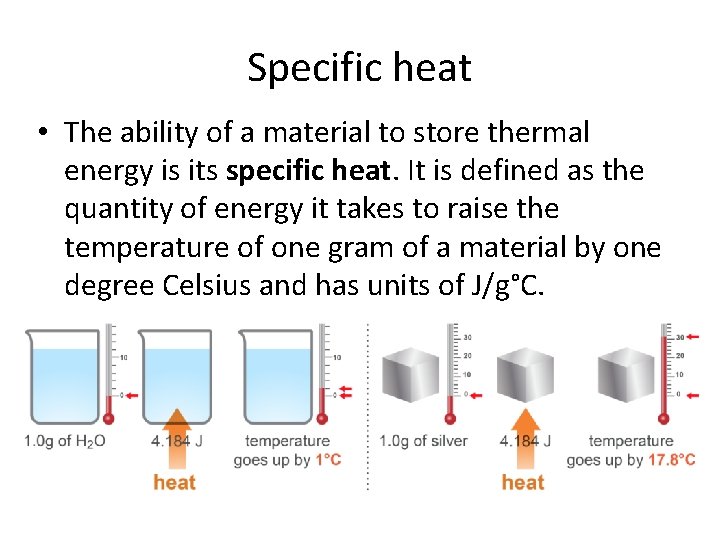
Specific heat • The ability of a material to store thermal energy is its specific heat. It is defined as the quantity of energy it takes to raise the temperature of one gram of a material by one degree Celsius and has units of J/g°C.

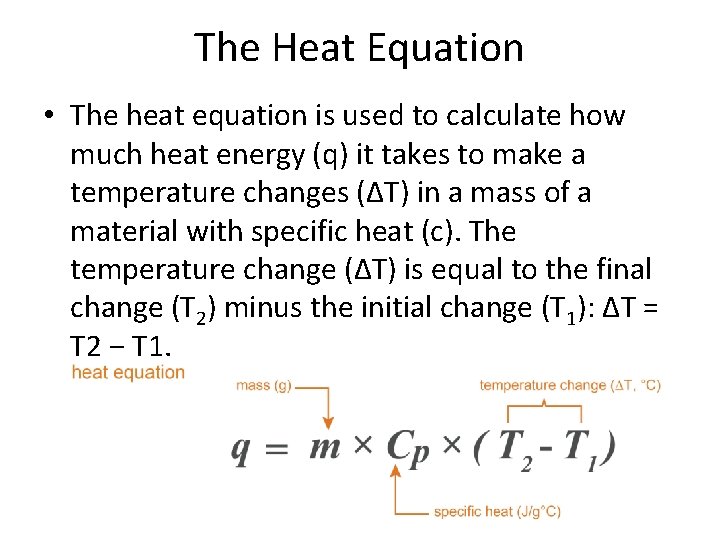
The Heat Equation • The heat equation is used to calculate how much heat energy (q) it takes to make a temperature changes (ΔT) in a mass of a material with specific heat (c). The temperature change (ΔT) is equal to the final change (T 2) minus the initial change (T 1): ΔT = T 2 − T 1.

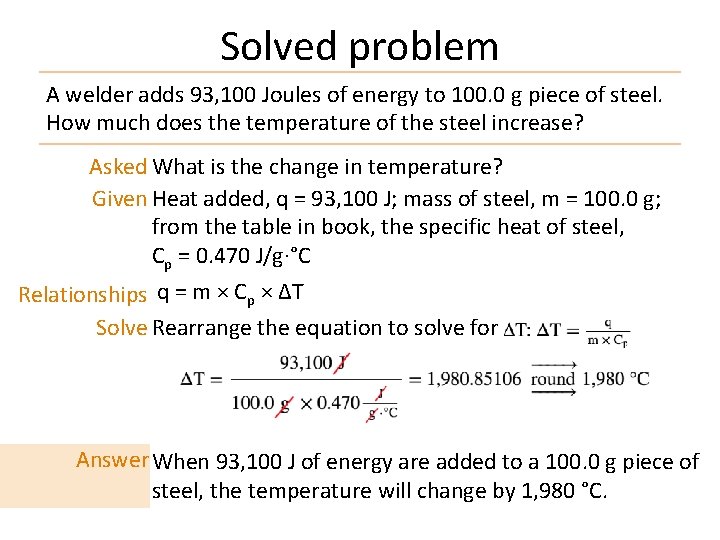
Solved problem A welder adds 93, 100 Joules of energy to 100. 0 g piece of steel. How much does the temperature of the steel increase? Asked What is the change in temperature? Given Heat added, q = 93, 100 J; mass of steel, m = 100. 0 g; from the table in book, the specific heat of steel, Cp = 0. 470 J/g·°C Relationships q = m × Cp × ΔT Solve Rearrange the equation to solve for Answer When 93, 100 J of energy are added to a 100. 0 g piece of steel, the temperature will change by 1, 980 °C.

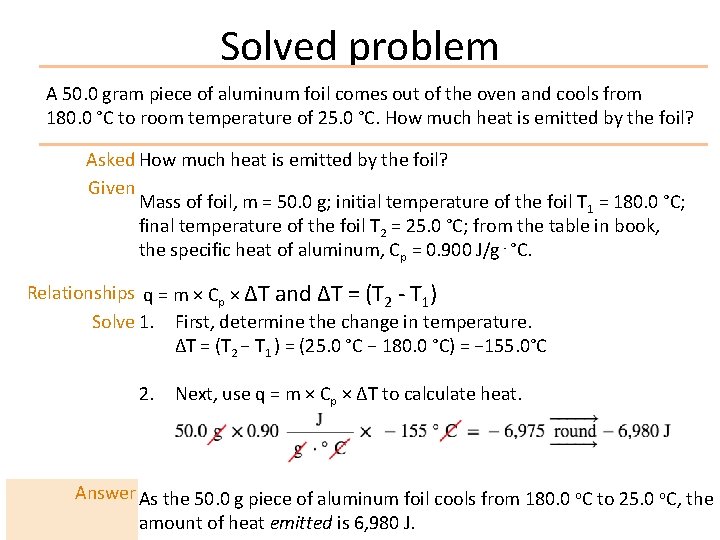
Solved problem A 50. 0 gram piece of aluminum foil comes out of the oven and cools from 180. 0 °C to room temperature of 25. 0 °C. How much heat is emitted by the foil? Asked How much heat is emitted by the foil? Given Mass of foil, m = 50. 0 g; initial temperature of the foil T 1 = 180. 0 °C; final temperature of the foil T 2 = 25. 0 °C; from the table in book, the specific heat of aluminum, Cp = 0. 900 J/g⋅°C. Relationships q = m × Cp × ΔT and ΔT = (T 2 - T 1) Solve 1. First, determine the change in temperature. ΔT = (T 2 − T 1 ) = (25. 0 °C − 180. 0 °C) = − 155. 0°C 2. Next, use q = m × Cp × ΔT to calculate heat. Answer As the 50. 0 g piece of aluminum foil cools from 180. 0 o. C to 25. 0 o. C, the amount of heat emitted is 6, 980 J.

Heat and energy conservation • The first law of thermodynamics applies whether you are solving for energy transmitted to or from a system or its surroundings • If energy can neither be created nor destroyed, the amount of heat given off by a system equals the amount of heat absorbed by its surroundings

Energy and food • We cannot directly measure the energy you get from eating food. • The amount of energy your body contains before you eat a food cannot be determined. Therefore, the amount of energy you gain cannot be determined. • So scientists use water to catch the energy released when foods are burned in a device called a calorimeter.

Calories vs. calories • There are two types of calories. One thousand lower case calories are equal to one upper case Calorie (1, 000 cal = 1 Cal = 1 kcal). • Food is labeled in upper case Calories. • This notation is confusing, so sometimes we refer to uppercase Calories as kilocalories and write it as kcal.

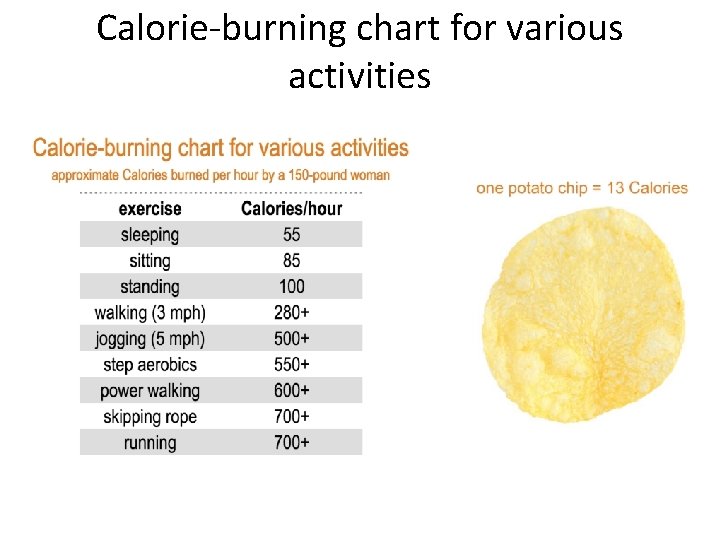
Calorie-burning chart for various activities

Solved problem How long will it take to walk off 13 Calories in a single potato chip? Given 13 Calories Relationships Walking requires 280 Cal/hr Solve Answer Walking 2. 8 mins will burn off the calories in a 13 -Calorie potato chip.

Post-assessment • Why are some regions of a phase change diagram flat?

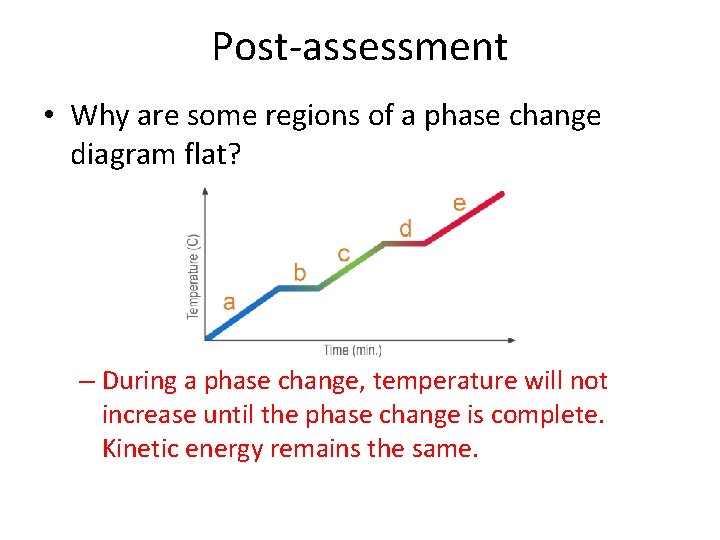
Post-assessment • Why are some regions of a phase change diagram flat? – During a phase change, temperature will not increase until the phase change is complete. Kinetic energy remains the same.

Post-assessment • How do you know whether energy is moving into a system or out of a system?

Post-assessment • How do you know whether energy is moving into a system or out of a system? – You have to think about what kind of change the system is undergoing. If the change only happens when the system loses energy, then energy must leave the system. If the change only happens if energy is added, then energy is going into a system.