Solid State Physics 22 lectures 6 hours of



- Slides: 10

Solid State Physics • • 22 lectures 6 hours of practical crystallography 6 hours of experiments on X-ray diffraction 6 hours of thin film deposition or thermistor properties • Assesement: 3 course works (10%), laboratory (20%) exams (70%)

Solid State Physics • Recommended text books: A Guinier& R Jullien The Solid State: From Superconductors to Superalloys, OUP 1989 R Turton : The Physics of Solids, OUP 2000 C Kittel: Introduction to Solid State Physics, Wiley&Sons, 1996

Crystals and amorphous materials Crystals: solid polyhedral form, whose surface may be regular and symmetric Steno’s law (1669): In all crystals of the same substance (and phase) the angles between corresponding faces are the same. Amorphous – solids without long-range repetitive order (glasses, plastics, resins)

Crystals and amorphous materials Metals – pure or combination of metallic elements. Non-localised electrons, good conductors of electricity and heat, strong, deformable Au, Ag, Al, Ni, Cr… Ceramics – compounds of metallica nad non-metallic elements: oxides, nitrides, carbides. Hard and resistant to high temperature and chemical attack. YBa. Cu. O… Semiconductors – electrical properties intermediate between metals and insulators Si, Ge, P, As, S Polymers – long chain-like molecules composed of hydrogen and nonmetallic elements. Low density, very flexible Composites – more than one type of material with a combination of properties, e. g. fibreglass=glass fiber in polymetric material

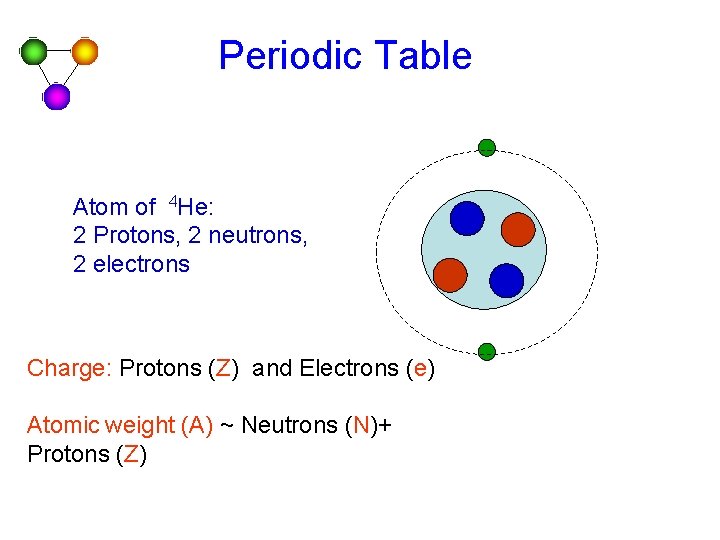
Periodic Table Atom of 4 He: 2 Protons, 2 neutrons, 2 electrons Charge: Protons (Z) and Electrons (e) Atomic weight (A) ~ Neutrons (N)+ Protons (Z)

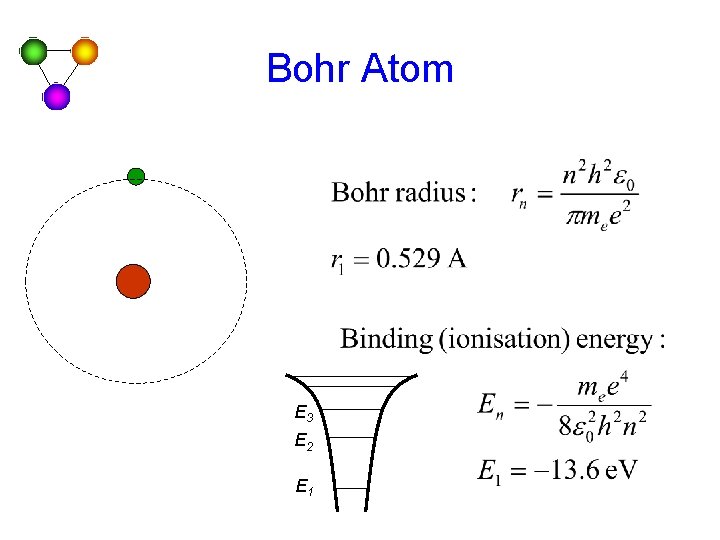
Bohr Atom E 3 E 2 E 1

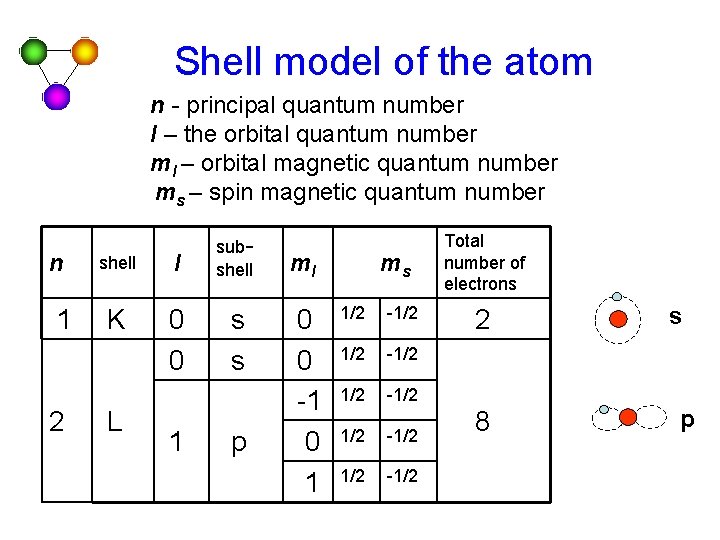
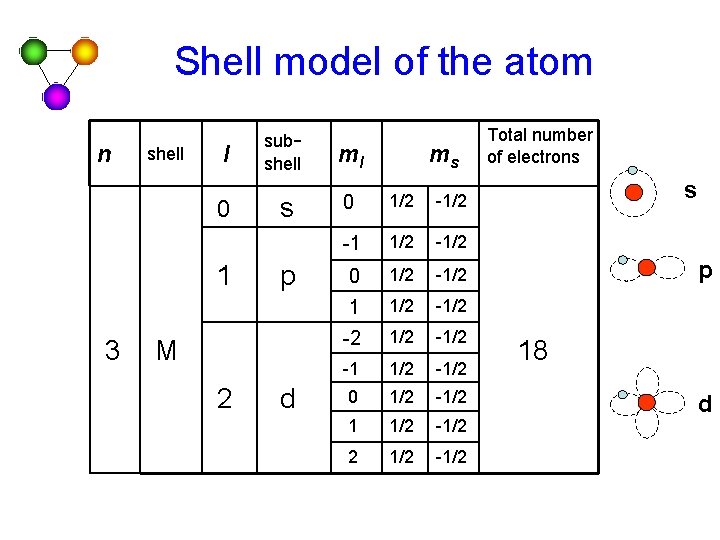
Shell model of the atom n - principal quantum number l – the orbital quantum number ml – orbital magnetic quantum number ms – spin magnetic quantum number n 1 2 shell l subshell K 0 0 s s L 1 p ml 0 0 -1 0 1 ms 1/2 -1/2 1/2 -1/2 Total number of electrons 2 8 s p

Shell model of the atom n shell l 0 1 3 subshell ml s 0 1/2 -1/2 0 1/2 -1/2 1 1/2 -2 1/2 -1/2 0 1/2 -1/2 1 1/2 -1/2 2 1/2 -1/2 p M 2 d ms Total number of electrons s p 18 d

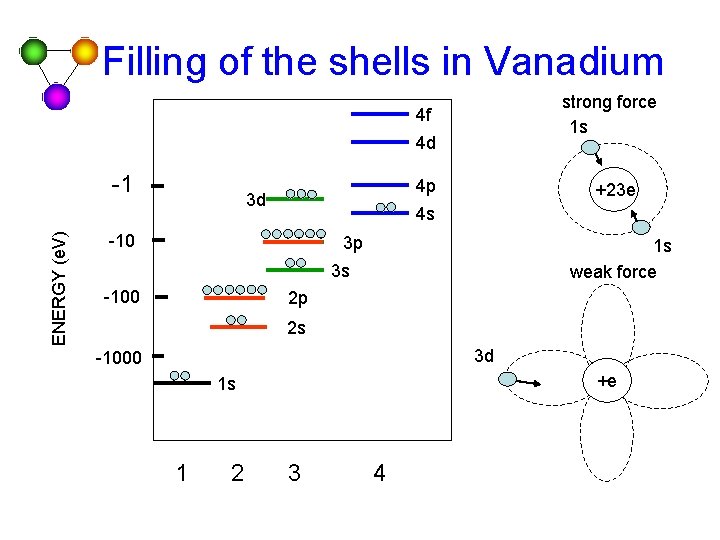
Filling of the shells in Vanadium strong force 1 s 4 f 4 d ENERGY (e. V) -1 4 p 3 d +23 e 4 s -10 3 p 1 s weak force 3 s -100 2 p 2 s 3 d -1000 +e 1 s 1 2 3 4

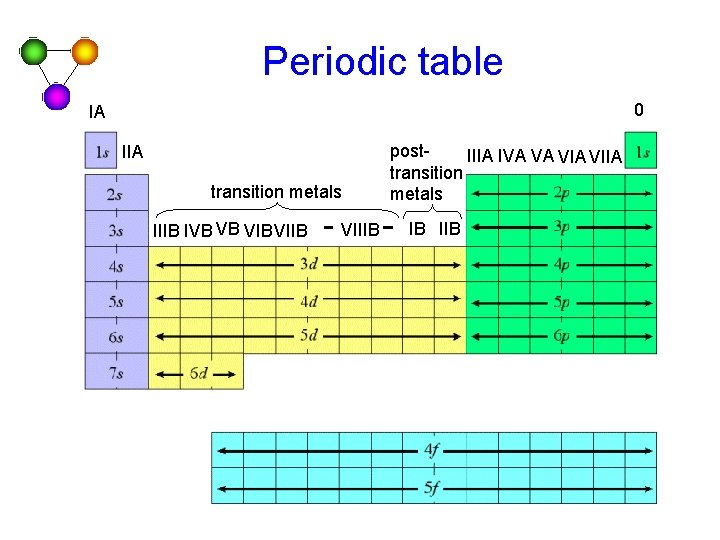
Periodic table 0 IA IIA transition metals IIIB IVB VB VIBVIIB VIIIB post. IIIA IVA VA VIIA transition metals IB IIB