Self emulsifying Drug Delivery System Preparation Evaluation Dr






- Slides: 10

Self emulsifying Drug Delivery System : Preparation & Evaluation Dr Trapti Saxena

STEP 1: SCREENING OF EXCIPIENTS Solubility Studies ØDissolve excess amount of drug in each of the solvents and put the samples in orbital shaker for 3 days. ØCentrifuge the equilibrated samples ØSuitably dilute the samples and analyze the solubility of drug. 2

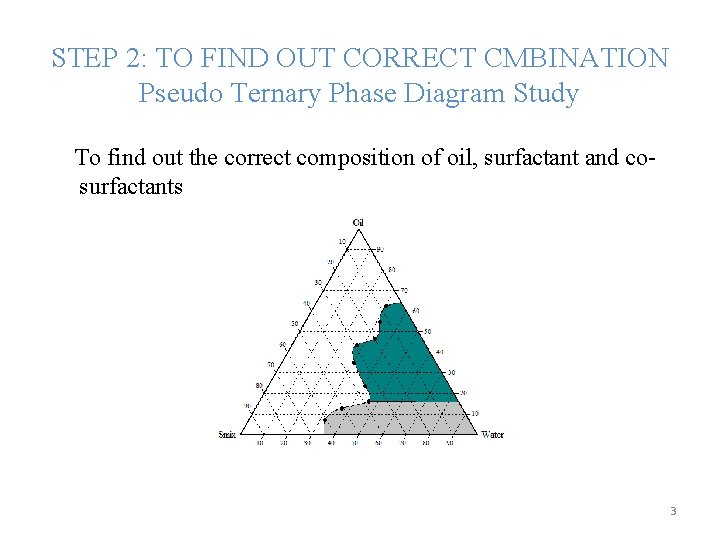
STEP 2: TO FIND OUT CORRECT CMBINATION Pseudo Ternary Phase Diagram Study To find out the correct composition of oil, surfactant and cosurfactants 3

STEP 3: ADDITION OF DRUG üRequired amount of drug is loaded to SMEDDS. üGentle stirring üSolidification using solid carrier : for solid SMEDDS 4

Evaluation of Self-microemulsifying System Self-emulsification Time Add SMEDDS to 20 m. L of water and 300 m. L of 0. 1 N HCl Monitor the tendency to emulsify spontaneously and Final appearance 5

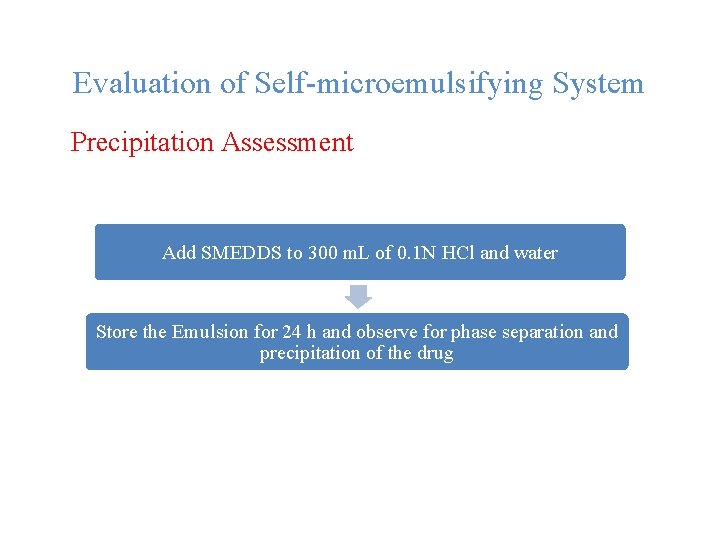
Evaluation of Self-microemulsifying System Precipitation Assessment Add SMEDDS to 300 m. L of 0. 1 N HCl and water Store the Emulsion for 24 h and observe for phase separation and precipitation of the drug

Evaluation of Self-microemulsifying System Robustness to Dilution 50 X, 100 X and 1000 X dilution of SMEDDS Emulsion - store for 24 h and observe for phase separation and precipitation of the drug 7

In vitro Drug Release Studies from Self-emulsifying Formulation • Pure drug and SMEDDS formulations - fill in hard gelatin capsules of size ‘ 0’ • Dissolution apparatus USP II is used • The samples is analyzed. 8

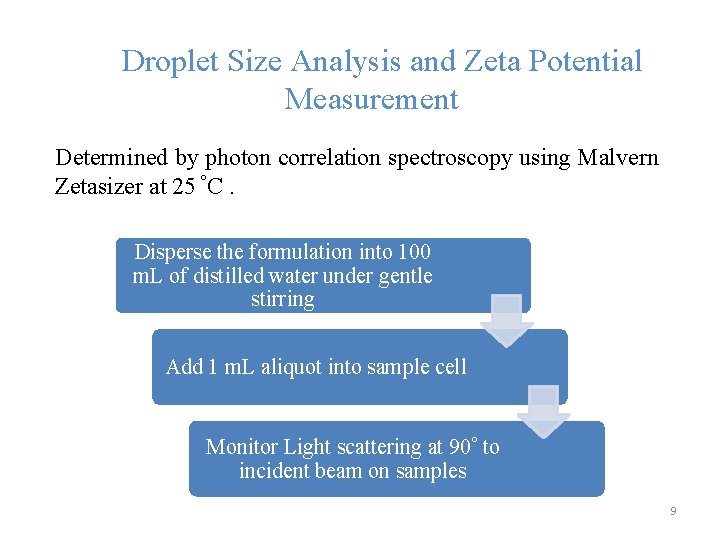
Droplet Size Analysis and Zeta Potential Measurement Determined by photon correlation spectroscopy using Malvern Zetasizer at 25 °C. Disperse the formulation into 100 m. L of distilled water under gentle stirring Add 1 m. L aliquot into sample cell Monitor Light scattering at 90° to incident beam on samples 9

Evaluation of Self-microemulsifying System • Viscosity : by brookfield viscometer • Refractive index: by refractometer and compared it with water (1. 333). • Percentage transmittance: measured at particular wavelength using Uv spectrophotometer. • Turbidimetric evaluation: by Nephelometer to monitor the growth of emulsification.