Section Plant Soil Science Unit Unit 7 Soil








- Slides: 16

Section: Plant & Soil Science Unit: Unit 7: Soil Management Lesson Title Lesson 1: Introduction to Soil Fertility and p. H with Soil Sampling Lab

Slide #2 Most popular macronutrients in soil • Nitrogen------N • Phosphorus-----P (usually in the form of P 2 O 5) • Potassium-------K (usually in the form of K 2 O)

Slide #3 What does nitrogen do? • Most popular macronutrient • It give a plant it’s green color • Assists with rapid growth and vigor • Promotes seed and fruit development • Increases protein and yield

Slide #4 Phosphorous (P 2 O 5) is important why? • Aids seedlings to germinate • Very important in the early stages of the crop • Stimulates root growth

Slide #5 What does Potassium (K 20) do? • Responsible for production of carbohydrates in the plant, • Produces plumper seeds • Acts as the water valve for letting water in and out of the plant.

Slide #6 What are fertilizers? • Help plants get the nutrients they need by adding deficient materials to the soil. • It makes nutrients more available to the plant to get what it needs

Slide #7 How can we find out what is in the soil? • Visual Inspection—not accurate • Tissue Cultures—accurate but expensive • Soil Test—most widely accepted form of testing

Slide #8 What forms are fertilizers found? • Dry (granules) • Liquid (usally diluted with water) • Gas (anhydrous ammonia) • Solids (manure) • Spikes (concentrated quantities placed in ground allowed to dissipate through soil)

Slide #9 Reading a bag of fertilizer N -P- K 20 -15 -10 • 20% Nitrogen • 15% Phosphorus • 10% Potassium

Slide #10 How many pounds are in there? Assume the bag is 100 pounds • 20% Nitrogen means there is 20 pounds of Nitrogen • Phosphorus in the form of P 2 O 5 15% X. 44=6. 6 lbs • Potassium in the form of K 2 O 10% x. 83= 8. 3 lbs For bags smaller than 100 lbs, multiply your pounds by the pounds in the sack then divide by 100. Example of 50 lb sack. 8. 3 x 50/100 = 4. 15 lbs of Potassium

Slide #11 Math problems · A 50# bag of 10 -10 -10 #N #P #K__ · A 50 # bag of 10 -20 -15 #N #P #K__ · A 100# bag of 20 -10 -0 #N #P #K__

Slide #12 Answers to math problems · A 50# bag of 10 -10 -10 #N 5 #P 2. 2 #K 4. 15 · A 50 # bag of 10 -20 -15 #N 5 #P 4. 4 #K 6. 225 · A 100# bag of 20 -10 -0 #N 20 #P 4. 4 #K 0

Slide #13 What is p. H? p. H is the scale we use to measure acidity (sour) to basic (bitter). What is the range of p. H? You are right the range is 0 -14.

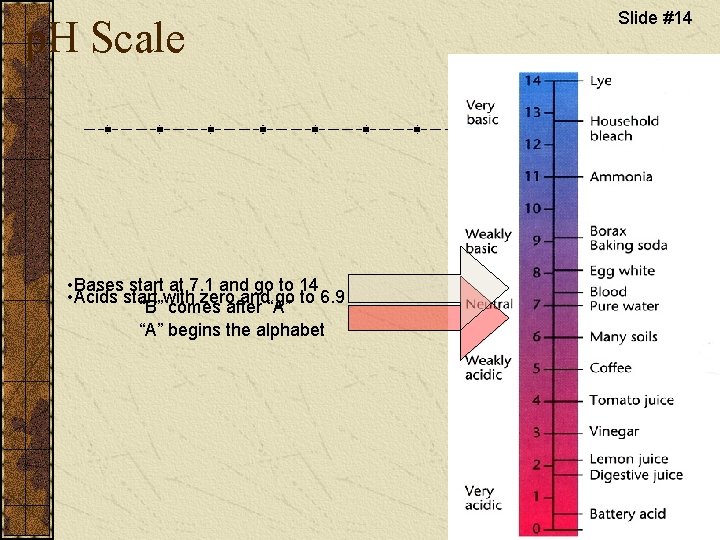
p. H Scale • Bases start at 7. 1 and go to 14 • Acids start with zero and go to 6. 9 “B” comes after “A” begins the alphabet Slide #14

Slide #15 What does p. H measure? p. H is the measure of how an item reacts with water. If it gives off hydrogen ions (H+) it is an acid. An example would be hydrochloric acid. When it meets water it forms hydrogen ions and chloride ions. A base give off hydroxide ions when reacting with water. Sodium hydroxide is a base because it forms sodium ions and hydroxide ions when mixed with water.

Slide #16 Why do we need to know p. H? Knowing the p. H of our soil is important for two reasons: It allows us to figure what amendments we might need to make the soil more efficient for the crop we are to be growing It also determines nutrient availability (some nutrients become unavailable to plants at low or high p. H levels)