Section 1 Energy can change form and flow

- Slides: 13

Section 1: Energy can change form and flow, but it is always conserved. K What I Know W What I Want to Find Out L What I Learned

• 11(B) Understand the law of conservation of energy and the processes of heat transfer. • 11(A) Understand energy and its forms, including kinetic, potential, chemical, and thermal energies. • 11(D) Perform calculations involving heat, mass, temperature change, and specific heat. • 2(G) Express and manipulate chemical quantities using scientific conventions and mathematical procedures, including dimensional analysis, scientific notation, and significant figures. Copyright © Mc. Graw-Hill Education Energy

Essential Questions • What is energy? • How do potential and kinetic energy differ? • How can chemical potential energy be related to the heat lost or gained in chemical reactions? Copyright © Mc. Graw-Hill Education Energy

Vocabulary Review New • temperature • energy • law of conservation of energy • chemical potential energy • heat • calorie • joule • specific heat Copyright © Mc. Graw-Hill Education Energy

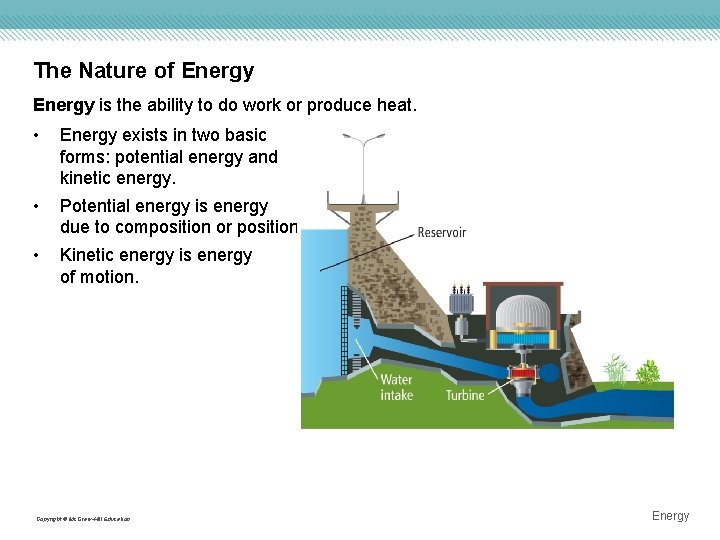
The Nature of Energy is the ability to do work or produce heat. • Energy exists in two basic forms: potential energy and kinetic energy. • Potential energy is energy due to composition or position. • Kinetic energy is energy of motion. Copyright © Mc. Graw-Hill Education Energy

The Nature of Energy The law of conservation of energy states that in any chemical reaction or physical process, energy can be converted from one form to another, but it is neither created nor destroyed—also known as the first law of thermodynamics. Chemical potential energy is energy stored in a substance because of its composition. • Chemical potential energy is important in chemical reactions. Heat is energy that is in the process of flowing from a warmer object to a cooler object. • q is used to symbolize heat. Copyright © Mc. Graw-Hill Education Energy

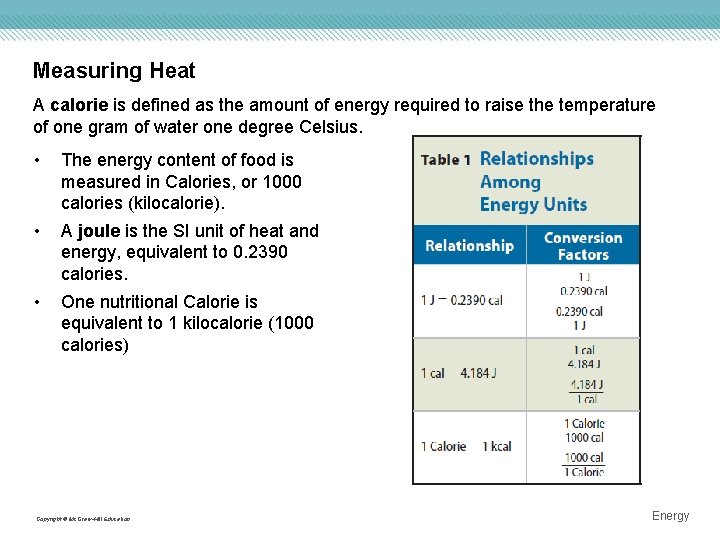
Measuring Heat A calorie is defined as the amount of energy required to raise the temperature of one gram of water one degree Celsius. • The energy content of food is measured in Calories, or 1000 calories (kilocalorie). • A joule is the SI unit of heat and energy, equivalent to 0. 2390 calories. • One nutritional Calorie is equivalent to 1 kilocalorie (1000 calories) Copyright © Mc. Graw-Hill Education Energy

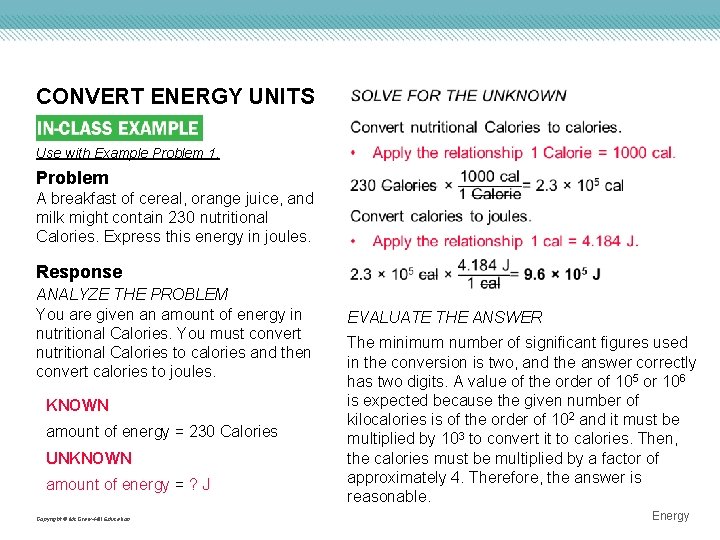
CONVERT ENERGY UNITS Use with Example Problem 1. Problem A breakfast of cereal, orange juice, and milk might contain 230 nutritional Calories. Express this energy in joules. Response ANALYZE THE PROBLEM You are given an amount of energy in nutritional Calories. You must convert nutritional Calories to calories and then convert calories to joules. KNOWN amount of energy = 230 Calories UNKNOWN amount of energy = ? J Copyright © Mc. Graw-Hill Education EVALUATE THE ANSWER The minimum number of significant figures used in the conversion is two, and the answer correctly has two digits. A value of the order of 105 or 106 is expected because the given number of kilocalories is of the order of 102 and it must be multiplied by 103 to convert it to calories. Then, the calories must be multiplied by a factor of approximately 4. Therefore, the answer is reasonable. Energy

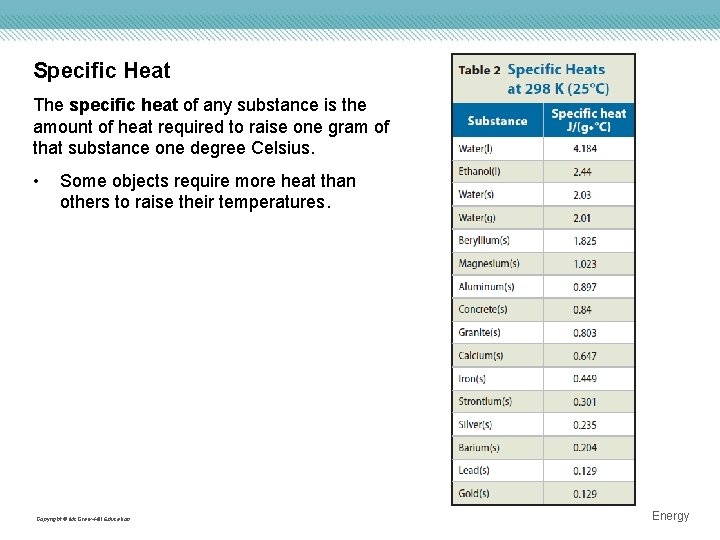
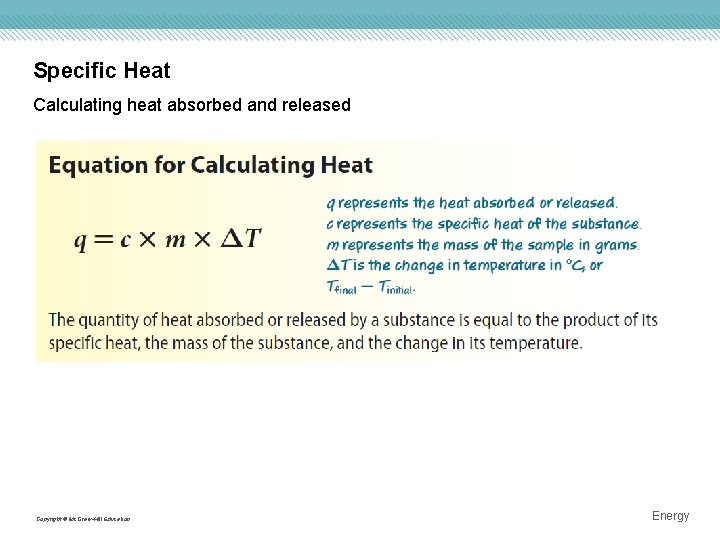
Specific Heat The specific heat of any substance is the amount of heat required to raise one gram of that substance one degree Celsius. • Some objects require more heat than others to raise their temperatures. Copyright © Mc. Graw-Hill Education Energy

Specific Heat Calculating heat absorbed and released Copyright © Mc. Graw-Hill Education Energy

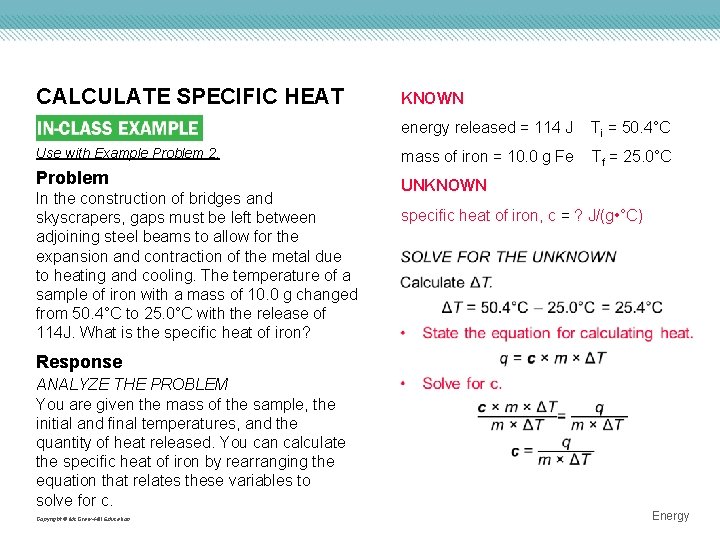
CALCULATE SPECIFIC HEAT KNOWN energy released = 114 J Ti = 50. 4°C Use with Example Problem 2. mass of iron = 10. 0 g Fe Problem Tf = 25. 0°C UNKNOWN In the construction of bridges and skyscrapers, gaps must be left between adjoining steel beams to allow for the expansion and contraction of the metal due to heating and cooling. The temperature of a sample of iron with a mass of 10. 0 g changed from 50. 4°C to 25. 0°C with the release of 114 J. What is the specific heat of iron? specific heat of iron, c = ? J/(g • °C) Response ANALYZE THE PROBLEM You are given the mass of the sample, the initial and final temperatures, and the quantity of heat released. You can calculate the specific heat of iron by rearranging the equation that relates these variables to solve for c. Copyright © Mc. Graw-Hill Education Energy

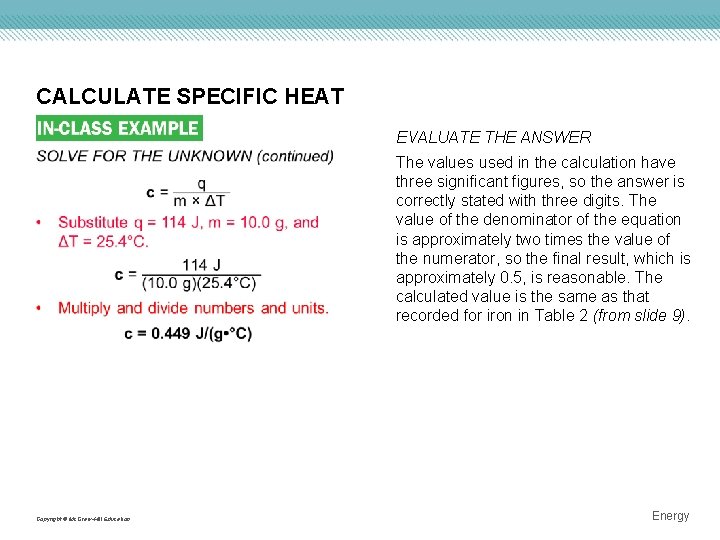
CALCULATE SPECIFIC HEAT EVALUATE THE ANSWER The values used in the calculation have three significant figures, so the answer is correctly stated with three digits. The value of the denominator of the equation is approximately two times the value of the numerator, so the final result, which is approximately 0. 5, is reasonable. The calculated value is the same as that recorded for iron in Table 2 (from slide 9). Copyright © Mc. Graw-Hill Education Energy

Review Essential Questions • What is energy? • How do potential and kinetic energy differ? • How can chemical potential energy be related to the heat lost or gained in chemical reactions? Vocabulary • energy • law of conservation of energy • chemical potential energy Copyright © Mc. Graw-Hill Education • heat • calorie • joule • specific heat Energy