Salts in Solution Mrs Coyle Solutions of Salts





- Slides: 12

Salts in Solution Mrs. Coyle

Solutions of Salts -Strong Acids and Strong Bases • Produce a neutral solution (p. H=7) • Example: HCl + Na. OH Strong Acid Strong Base Na. Cl + H 2 O Neutral Solution

Solutions of Salts -Strong Acids and Weak Bases • Produce an acidic solution (p. H<7) • Example: HCl + NH 3 Strong Acid Weak Base NH 4 Cl + H 2 O Acidic Solution

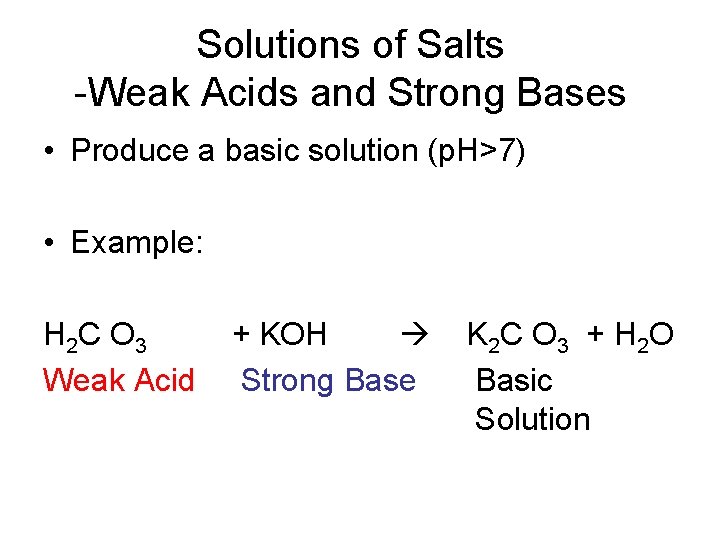
Solutions of Salts -Weak Acids and Strong Bases • Produce a basic solution (p. H>7) • Example: H 2 C O 3 Weak Acid + KOH Strong Base K 2 C O 3 + H 2 O Basic Solution

Solutions of Salts -Weak Acids and Weak Bases • The p. H of their salt’s solution depends on their relative strength.

Summary Strong Acid + Strong Base Neutral Solution Strong Acid + Weak Base Acidic Solution Weak Acid + Strong Base Basic Solution

Why does this happen? • Salt Hydrolysis • Ions of the dissociated salt, remove or donate H+ , to the solution.

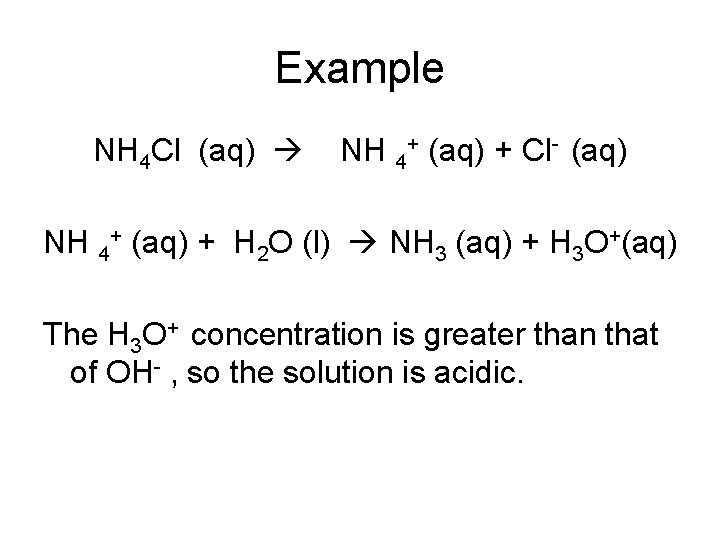
Example NH 4 Cl (aq) NH 4+ (aq) + Cl- (aq) NH 4+ (aq) + H 2 O (l) NH 3 (aq) + H 3 O+(aq) The H 3 O+ concentration is greater than that of OH- , so the solution is acidic.

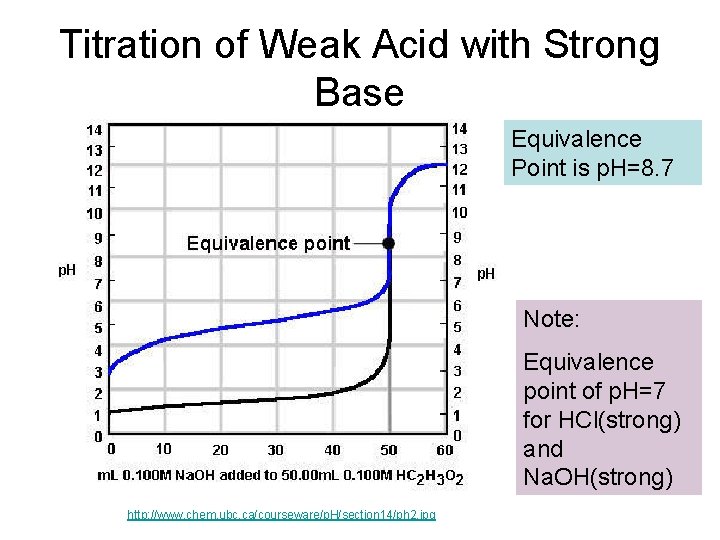
Titration of Weak Acid with Strong Base Equivalence Point is p. H=8. 7 Note: Equivalence point of p. H=7 for HCl(strong) and Na. OH(strong) http: //www. chem. ubc. ca/courseware/p. H/section 14/ph 2. jpg

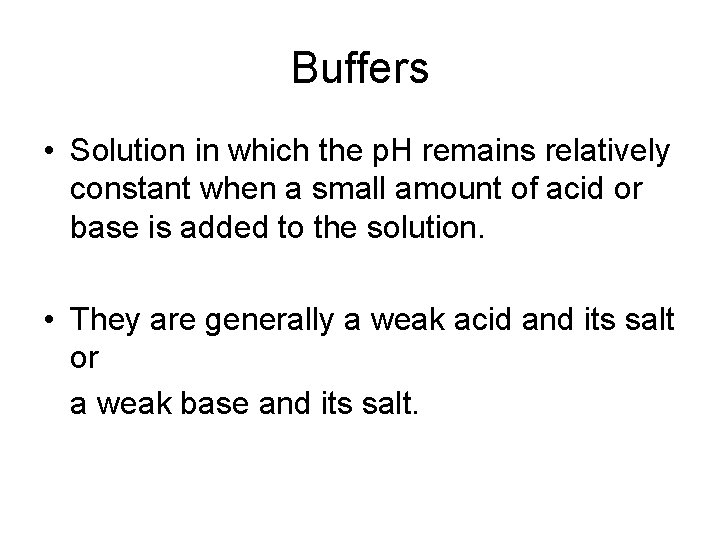
Buffers • Solution in which the p. H remains relatively constant when a small amount of acid or base is added to the solution. • They are generally a weak acid and its salt or a weak base and its salt.

Examples of Buffers • H 2 CO 3 and the salt of HCO 3 - (in human blood) • NH 3 and the salt of NH 4+

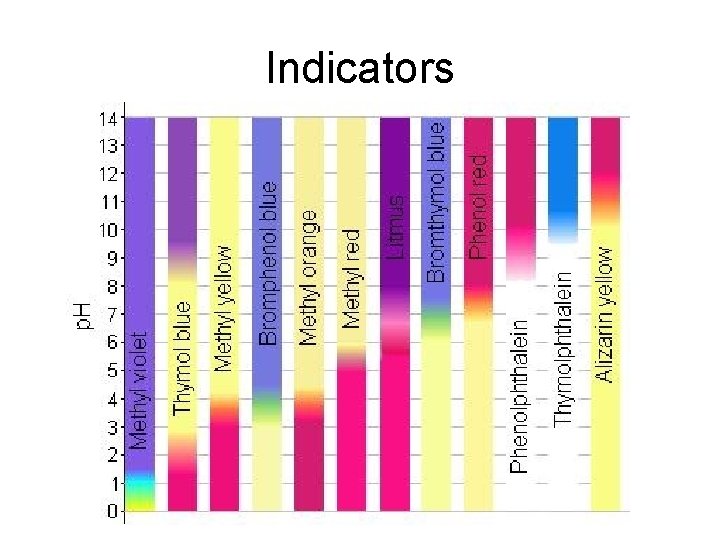
Indicators