The Solution Process Chemistry Mrs Coyle Solution A





















- Slides: 32

The Solution Process Chemistry Mrs. Coyle

Solution A homogeneous mixture. Stainless Steel (Fe, Cr, Ni) n One phase. n

Solute, Solvent • Solute—the substance being dissolved. • Example: When you dissolve Cu. Cl 2 in water, Cu. Cl 2 is the solute. • Solvent- the substance that dissolves the solute. • Example: water

Aqueous Solution • A solution that has water as the solvent. • Possible substances that can dissolve in water: ¨ Ionic compounds ¨ Polar covalent compounds

Solvation n The surrounding of solute particles by solvent particles.

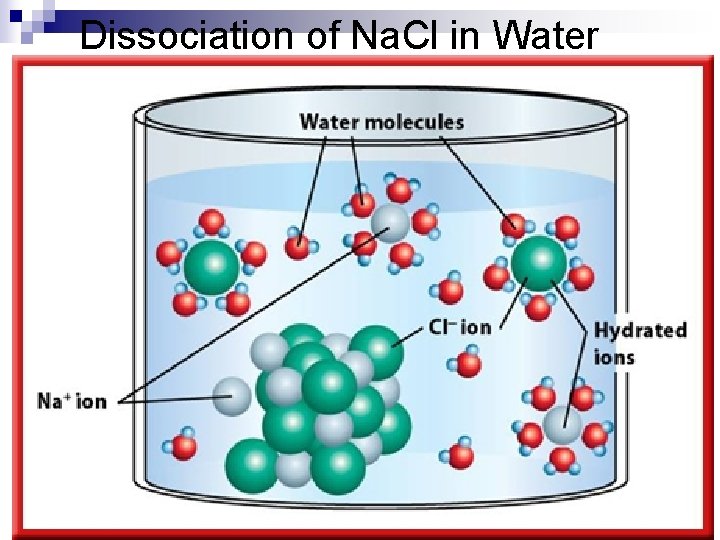
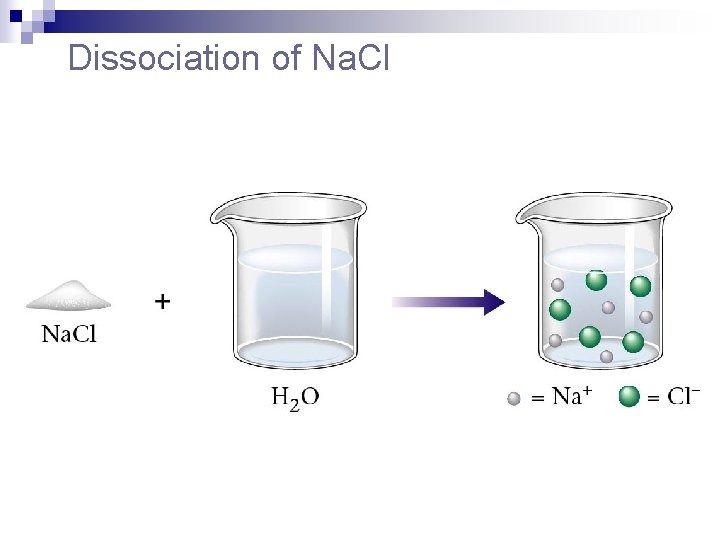
Dissociation of Ionic Compounds n the process by which an ionic compound separates into its ions as it dissolves.

Dissociation of Na. Cl in Water

Dissociation of Na. Cl

Movie Clip- Dissociation of Salt in Water http: //www. youtube. com/watch? v=EBf. Gc. TAJF 4 o

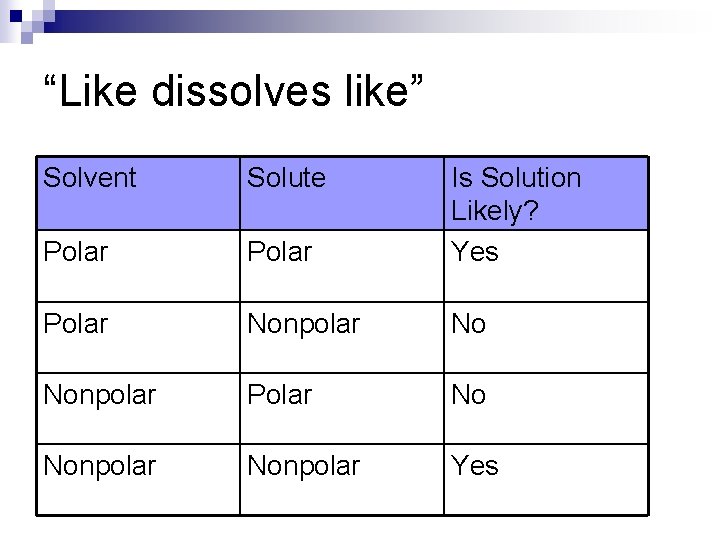
“Like dissolves like” Solvent Solute Polar Is Solution Likely? Yes Polar Nonpolar No Nonpolar Polar No Nonpolar Yes

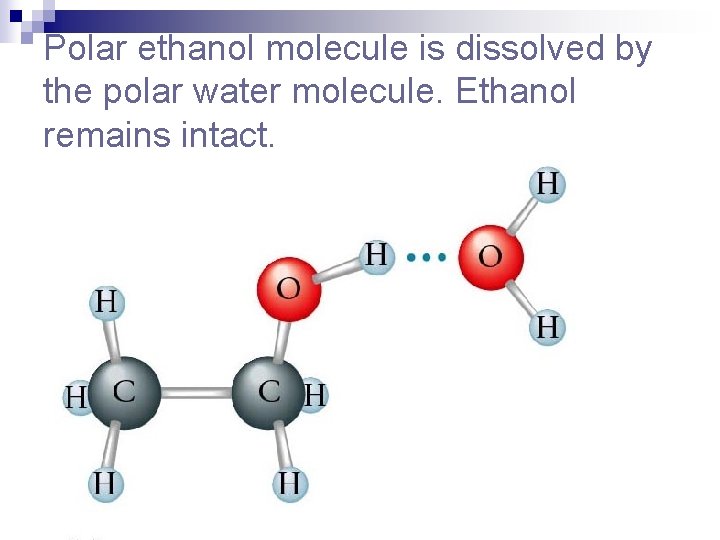
Polar ethanol molecule is dissolved by the polar water molecule. Ethanol remains intact.

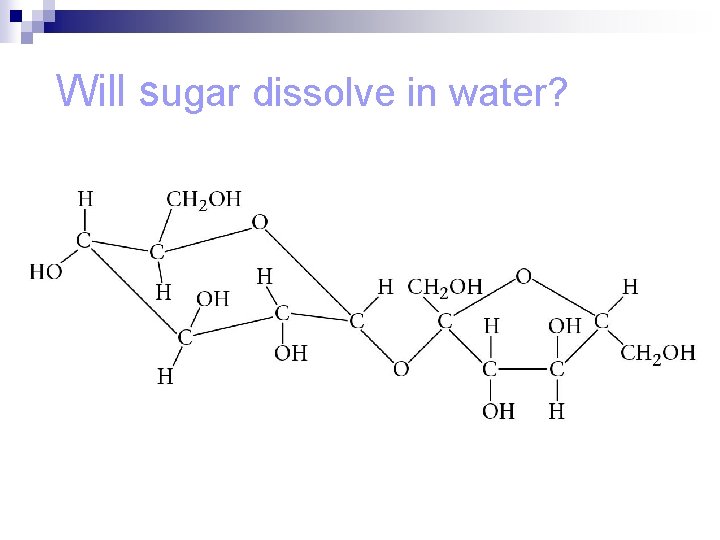
Will sugar dissolve in water?

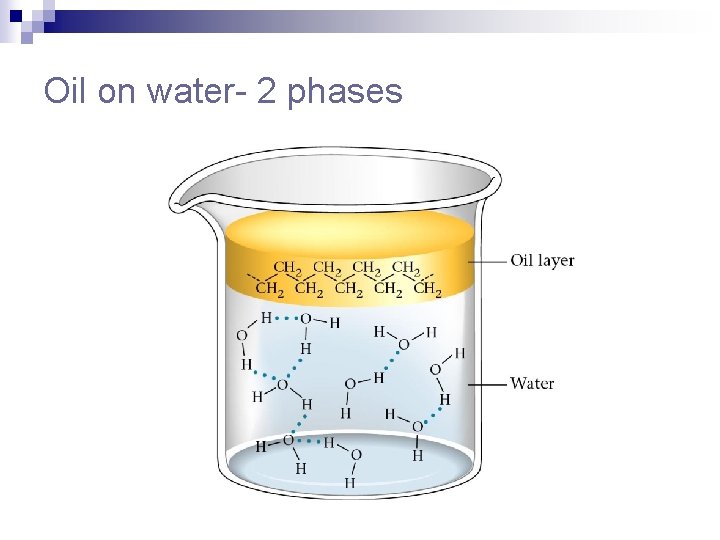
Will petroleum dissolve in water?

Oil on water- 2 phases

Will ionic compounds conduct electric current when dissolved in water? n Yes n Why?

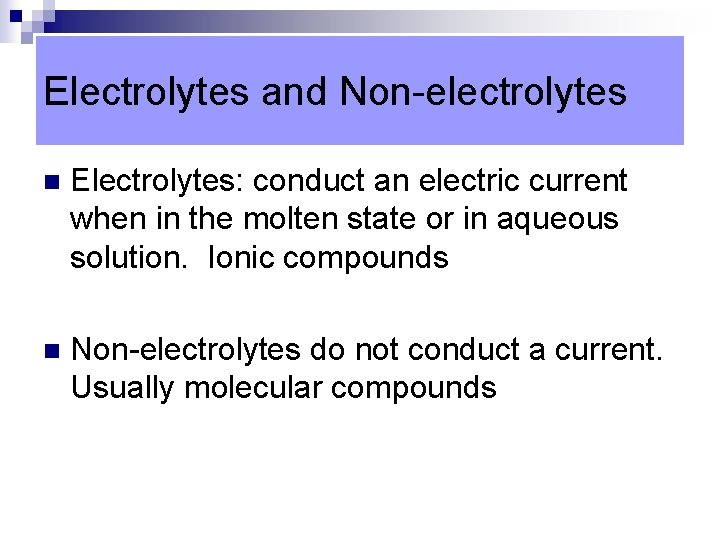
Electrolytes and Non-electrolytes n Electrolytes: conduct an electric current when in the molten state or in aqueous solution. Ionic compounds n Non-electrolytes do not conduct a current. Usually molecular compounds

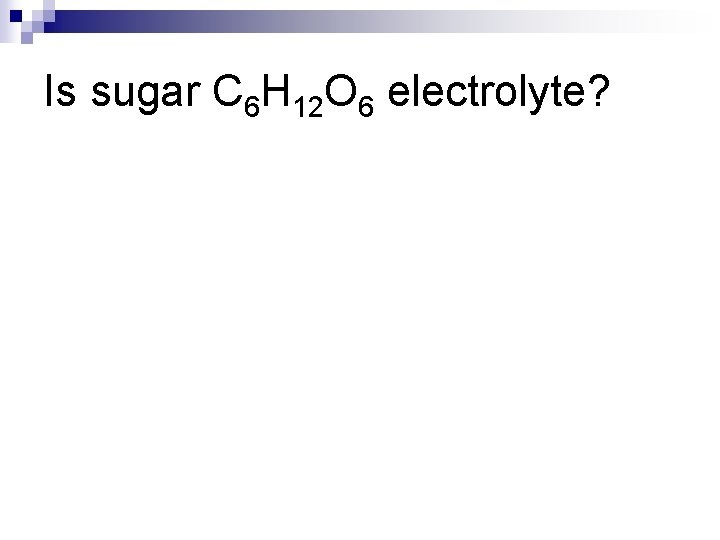
Is sugar C 6 H 12 O 6 electrolyte?

Do all electrolytes conduct electricity to the same degree? n Weak electrolytes: partially ionize in water and conduct electricity in solution poorly (ex. Ammonia) n Strong electrolytes: fully ionize in water and conduct electricity in solution strongly(ex. Na. Cl).

Hydrate: n A crystalline compound in which the ions are attached to one or more water molecules.

Example: n Cu. SO 4 • 5 H 2 O n copper(II) sulfate pentahydrate

Prefixes for naming Hydrates n mono- 1 n di 2 n tri 3 n tetra- 4 n penta- 5 n hexa- 6 n hepta- 7 n octa- 8 n nona- 9 n deca- 10

Analyzing Hydrates Simulation click on the link below: n http: //www. chem. iastate. edu/group/Green bowe/sections/projectfolder/flashfiles/stoic hiometry/empirical. html n

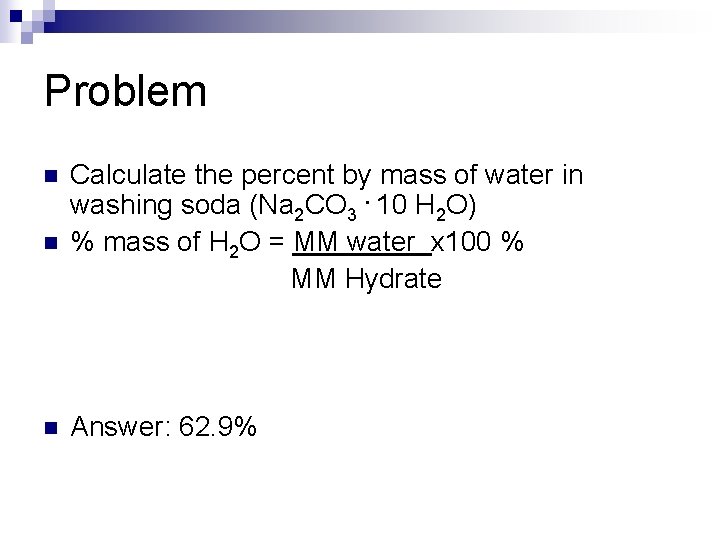
Problem n n n Calculate the percent by mass of water in washing soda (Na 2 CO 3. 10 H 2 O) % mass of H 2 O = MM water x 100 % MM Hydrate Answer: 62. 9%

Efflorescent Hydrates that have high vapor pressures compared to water. n When the vapor pressure of the surrounding is lower than the vapor pressure of the hydrate, the hydrate will lose its water; it effloresces. n

Hygroscopic Hydrates and Dessicants Hydrates that have a low vapor pressure compared to water. n These hydrates can absorb water from the air. n These can be used as dessicants (ex. Ca. SO 4). n

Deliquescent n Materials that absorb so much water that they will become wet (form solutions). Ex. Na. OH.

Part II Heterogeneous Aqueous Systems

Colloids and Suspensions n Heterogeneous Mixtures

Suspension n. A mixture whose particles are temporarily suspended in a medium, but eventually settle down. n Particle size>100 nm n Ex: dust in air.

Colloid n. A mixture whose particles (of size ~1 to ~100 nm) are dispersed through a continuous medium. (The word colloid means “glue-like”) n Heterogeneous because there are distinct phases. n Tyndall Effect: Scattering of light.

Tyndall Effect

Types of Colloids n Aerosol: liquid or solid in dispersed in gases (fog, smoke). n Foam: gas in liquid (whipped cream). n Emulsion: both substances are liquids (mayonnaise). n Sol: solid in liquid (jelly)