ROTARY LEAD RECYCLING VESSELS APRIL 2013 SCRAP LEAD





















- Slides: 33

ROTARY LEAD RECYCLING VESSELS APRIL 2013




SCRAP LEAD ACID BATTERIES PROCESSING COKE ADDITION REDUCES LEAD SULPHATE TO Pb. S AVOIDING EXCESSIVE SO 2 POLLUTION ADDITION OF CAST IRON (TURNINGS AND BORINGS) REDUCES LEAD SULPHIDE TO METALLIC LEAD AND IRON SULPHIDE. SODA ASH ADDITION ALSO TO DESULPHURIZE AND TO FLUIDIZE SLAG/MATTE BY-PRODUCT (REFRACTORY LINING SENSITIVE TO EXCESS SODA)

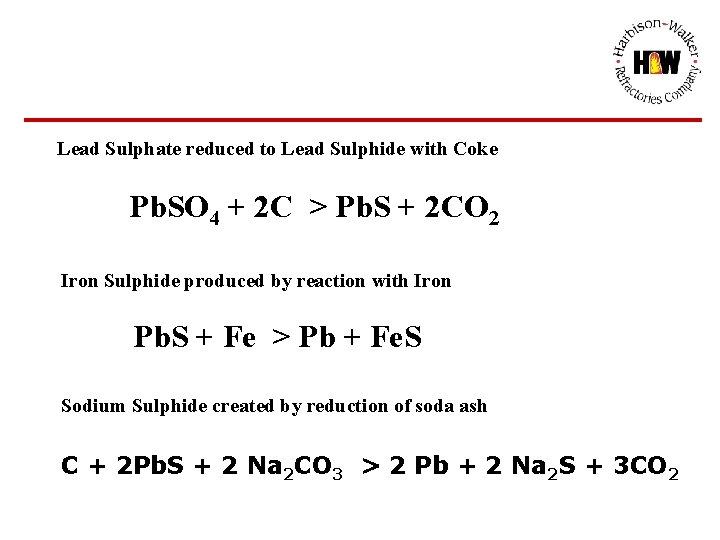
Lead Sulphate reduced to Lead Sulphide with Coke Pb. SO 4 + 2 C > Pb. S + 2 CO 2 Iron Sulphide produced by reaction with Iron Pb. S + Fe > Pb + Fe. S Sodium Sulphide created by reduction of soda ash C + 2 Pb. S + 2 Na 2 CO 3 > 2 Pb + 2 Na 2 S + 3 CO 2

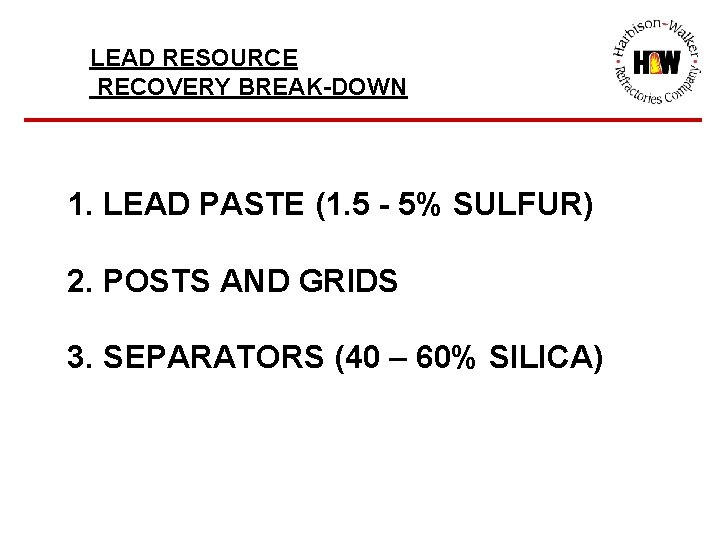
LEAD RESOURCE RECOVERY BREAK-DOWN 1. LEAD PASTE (1. 5 - 5% SULFUR) 2. POSTS AND GRIDS 3. SEPARATORS (40 – 60% SILICA)

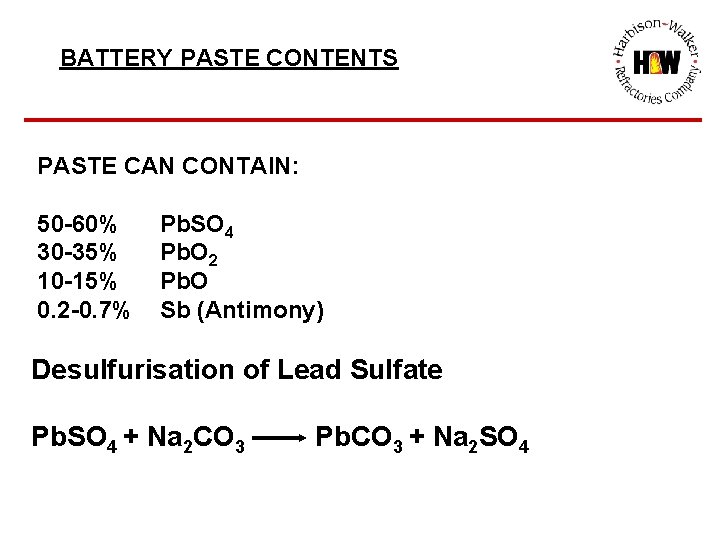
BATTERY PASTE CONTENTS PASTE CAN CONTAIN: 50 -60% 30 -35% 10 -15% 0. 2 -0. 7% Pb. SO 4 Pb. O 2 Pb. O Sb (Antimony) Desulfurisation of Lead Sulfate Pb. SO 4 + Na 2 CO 3 Pb. CO 3 + Na 2 SO 4

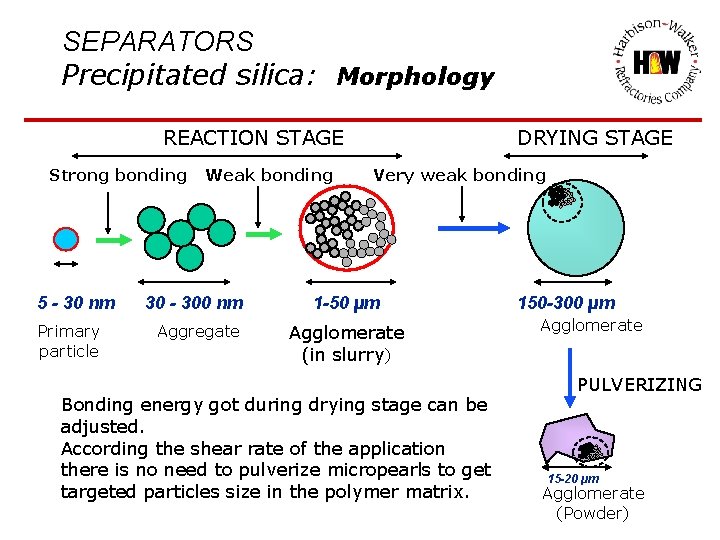
SEPARATORS Precipitated silica: Morphology REACTION STAGE Strong bonding 5 - 30 nm Primary particle Weak bonding DRYING STAGE Very weak bonding 30 - 300 nm 1 -50 µm Aggregate Agglomerate (in slurry) Bonding energy got during drying stage can be adjusted. According the shear rate of the application there is no need to pulverize micropearls to get targeted particles size in the polymer matrix. 150 -300 µm Agglomerate PULVERIZING 15 -20 µm Agglomerate (Powder)

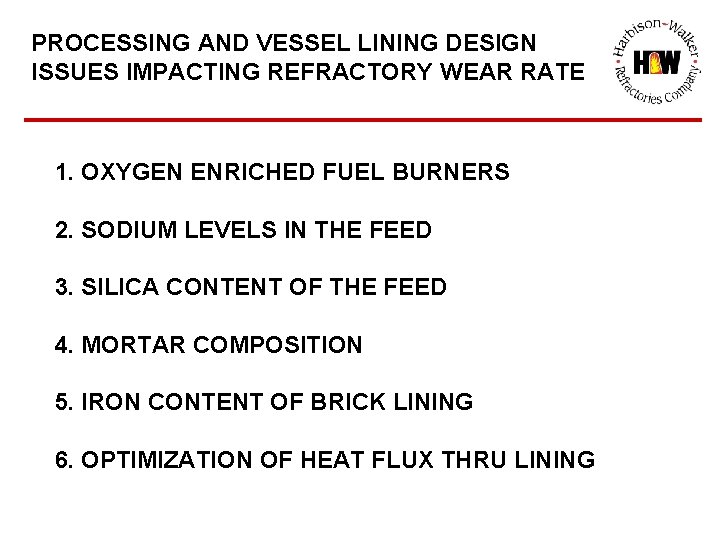
PROCESSING AND VESSEL LINING DESIGN ISSUES IMPACTING REFRACTORY WEAR RATE 1. OXYGEN ENRICHED FUEL BURNERS 2. SODIUM LEVELS IN THE FEED 3. SILICA CONTENT OF THE FEED 4. MORTAR COMPOSITION 5. IRON CONTENT OF BRICK LINING 6. OPTIMIZATION OF HEAT FLUX THRU LINING





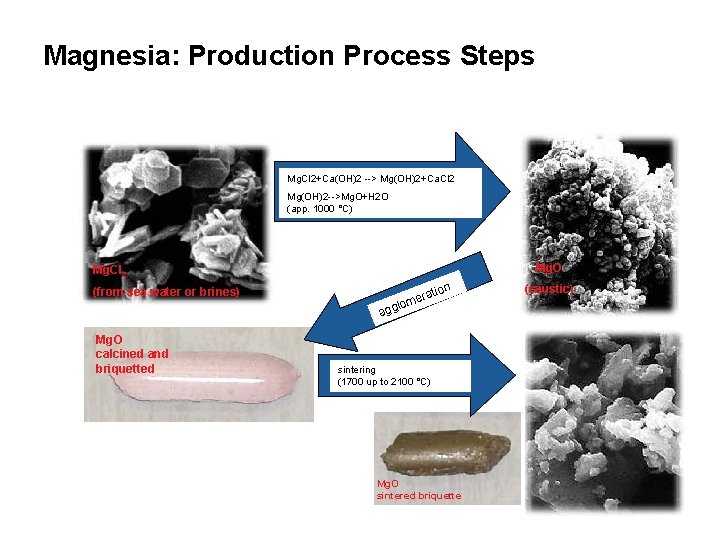
Magnesia: Production Process Steps Mg. Cl 2+Ca(OH)2 --> Mg(OH)2+Ca. Cl 2 Mg(OH)2 -->Mg. O+H 2 O (app. 1000 °C) Mg. O Mg. Cl 2 n atio (from sea water or brines) mer gglo a Mg. O calcined and briquetted sintering (1700 up to 2100 °C) Mg. O sintered briquette (caustic)

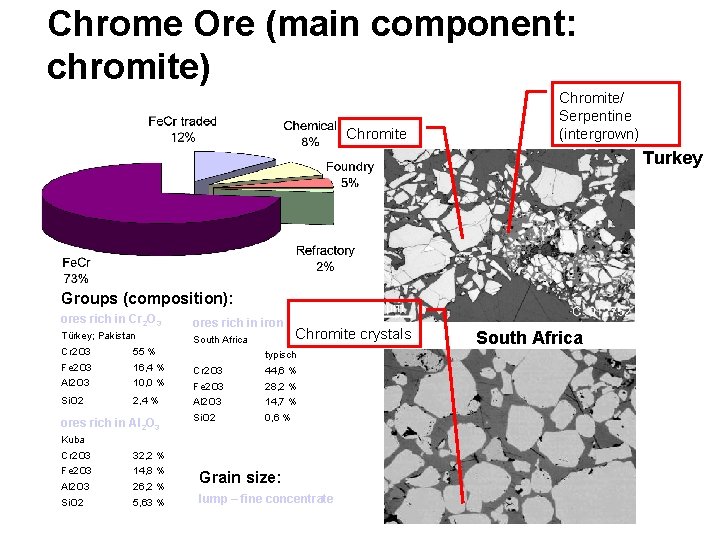
Chrome Ore (main component: chromite) Chromite/ Serpentine (intergrown) Turkey Groups (composition): ores rich in Cr 2 O 3 ores rich in iron Türkey; Pakistan South Africa Chromite crystals Cr 2 O 3 55 % Fe 2 O 3 16, 4 % Cr 2 O 3 44, 6 % Al 2 O 3 10, 0 % Fe 2 O 3 28, 2 % Si. O 2 2, 4 % Al 2 O 3 14, 7 % Si. O 2 0, 6 % ores rich in Al 2 O 3 typisch Kuba Cr 2 O 3 32, 2 % Fe 2 O 3 14, 8 % Al 2 O 3 26, 2 % Si. O 2 5, 63 % Grain size: lump – fine concentrate South Africa

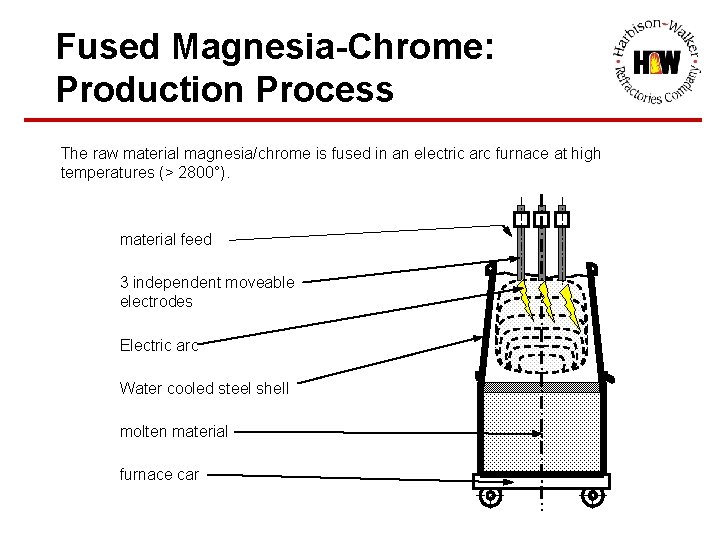
Fused Magnesia-Chrome: Production Process The raw material magnesia/chrome is fused in an electric arc furnace at high temperatures (> 2800°). material feed 3 independent moveable electrodes Electric arc Water cooled steel shell molten material furnace car

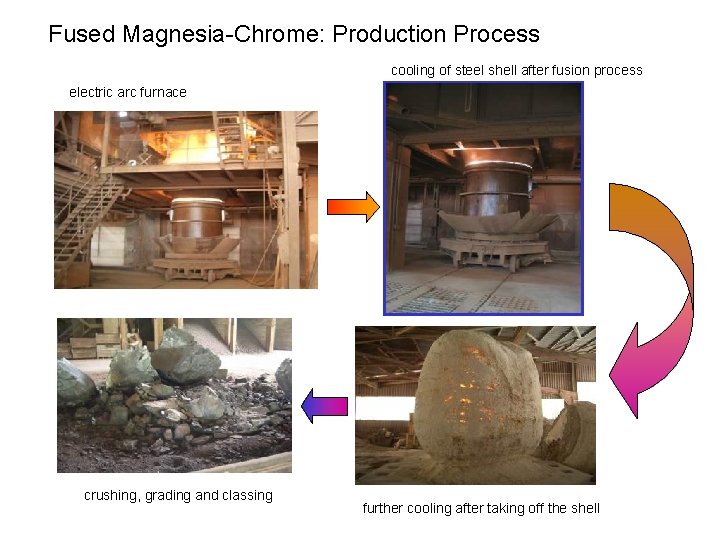
Fused Magnesia-Chrome: Production Process cooling of steel shell after fusion process electric arc furnace crushing, grading and classing further cooling after taking off the shell

10 mm. NARMAG 60 DB

10 mm. SUPER NARMAG 145

10 mm. SUPER NARMAG FG







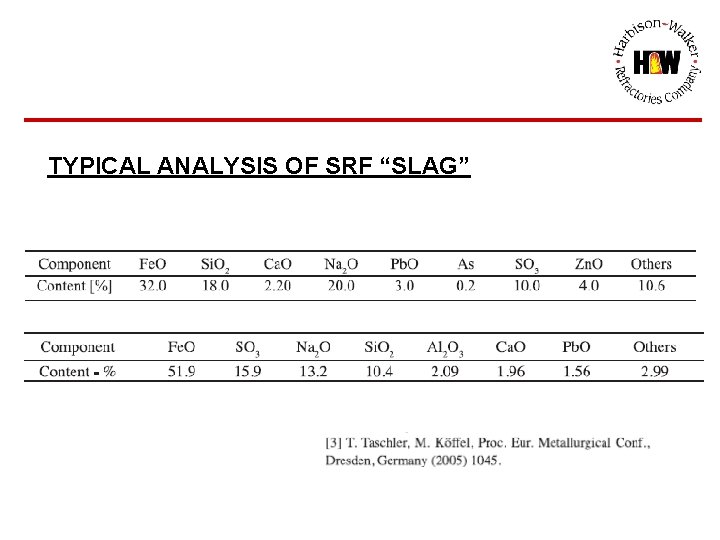
TYPICAL ANALYSIS OF SRF “SLAG”

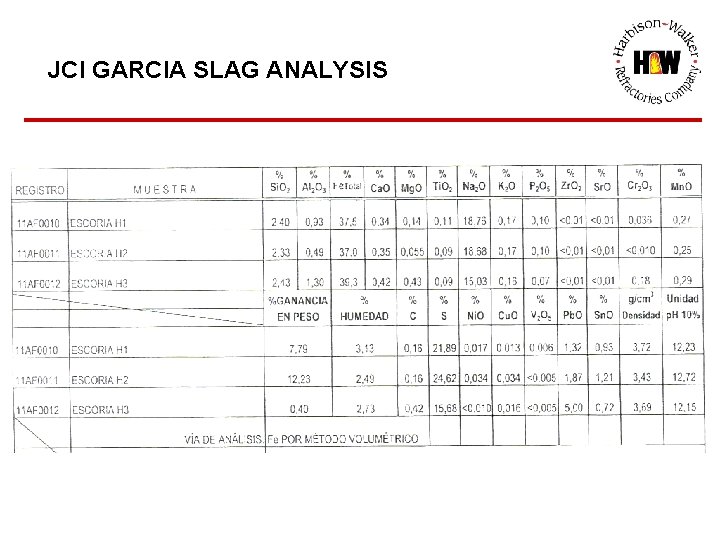
JCI GARCIA SLAG ANALYSIS


REDUCTION OF LEAD SULPHATE TO ELEMENTAL LEAD AND SODIUM/IRON SULPHIDE EUTECTIC SODA ASH, COKE AND IRON METALLICS REACT WITH LEAD SULPHATE/OXIDE TO PRODUCE SODIUM AND FERROUS SULPHIDE C + Pb. O + Pb. SO 4 > Pb + Pb. S + CO 2 Pb. S + Fe > Pb + Fe. S C + 2 Pb. S + 2 Na 2 CO 3 > 2 Pb + 2 Na 2 S + 3 CO 2


A. P. Green, Harbison-Walker, NARCO
 Certified scrap lead-acid batteries recycler
Certified scrap lead-acid batteries recycler Why did she take a tiny scrap of dough?
Why did she take a tiny scrap of dough? Lesson outline lesson 1 magnets and magnetic fields
Lesson outline lesson 1 magnets and magnetic fields Robin k wiener
Robin k wiener Cara menghitung scrap pada opc
Cara menghitung scrap pada opc Djj scrap connect
Djj scrap connect Steel scrap
Steel scrap Montgomery county scrap
Montgomery county scrap Electromagnetic application
Electromagnetic application The method of unit costing is adopted by
The method of unit costing is adopted by Spoilage, rework and scrap adalah
Spoilage, rework and scrap adalah Compared to an empty ship the same
Compared to an empty ship the same Blanking strip
Blanking strip Spoilage rework and scrap
Spoilage rework and scrap Ferrous scrap
Ferrous scrap Lead magnesium niobate
Lead magnesium niobate Bring your vessels not a few
Bring your vessels not a few Blood vessels
Blood vessels Dual laminate tanks
Dual laminate tanks In a great house there are vessels
In a great house there are vessels Eating together class 4 evs lesson plan
Eating together class 4 evs lesson plan Subcostalis and sternocostalis
Subcostalis and sternocostalis Alexandra schmalz
Alexandra schmalz Blood vessels
Blood vessels Sacred vessels used at mass
Sacred vessels used at mass Blood vessels adaptations
Blood vessels adaptations Mediastinum heart
Mediastinum heart What are capillaries
What are capillaries This is amazing grace hillsong
This is amazing grace hillsong Incisura anacrota
Incisura anacrota Bayoneting vessels glaucoma
Bayoneting vessels glaucoma Histological structure of blood vessels
Histological structure of blood vessels Is aortic atherosclerosis dangerous
Is aortic atherosclerosis dangerous Chapter 19 the circulatory or cardiovascular system
Chapter 19 the circulatory or cardiovascular system