Revision Chapter 1physical states Revision Chapter 1physical states










- Slides: 14

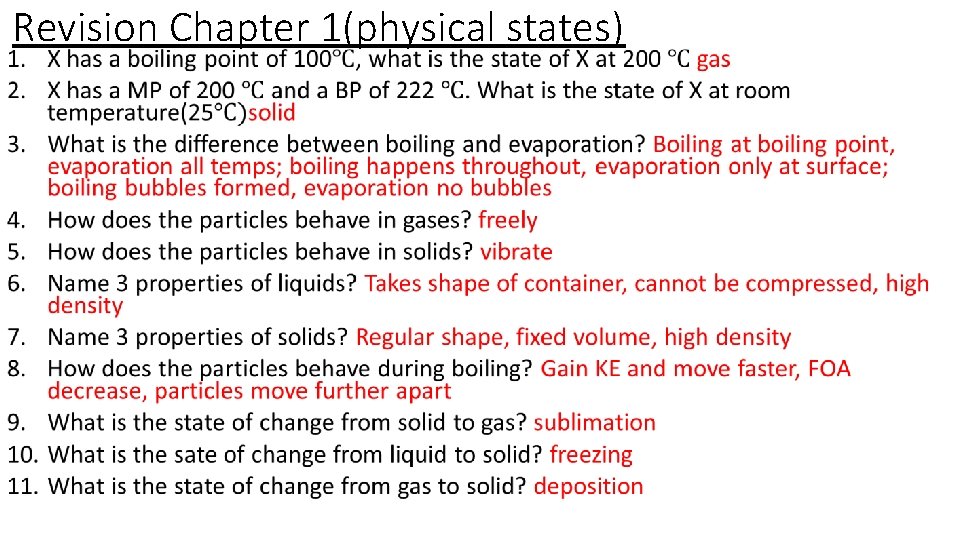
Revision Chapter 1(physical states) •

Revision Chapter 1(physical states) •

Revision Chapter 2 (metals, non-metals, and alloys) 1. 2. 3. 4. 5. 6. 7. On the periodic table, metals are arranged on the left or right side? Give 2 examples of metallic Define elements Define atoms Define alloys? Use a diagram to show the particles in alloys are arranged? Define 1. 2. 3. 4. 5. Malleable. Ductilestrong – Flexible- Tough- 8. How does metal conduct electricity? 9. How does metal conduct heat? 10. Explain why are non-metals not malleable? 11. Explain why are non-metals weak(not strong)and brittle

Revision Chapter 2 (metals, non-metals, and alloys) 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. On the periodic table, metals are arranged on the left or right side? left Give 2 examples of metallic element sodium potassium Define elements cannot be split into anything simpler Define alloys? mixture of two metals(or more) Use a diagram to show the particles in alloys are arranged? Define 1. 2. 3. 4. 5. Malleable-can be beaten into sheets Ductile-can be drawn into wires strong – can support heavy loads Flexible- can be bent easily Tough-can withstand impacts How does metal conduct electricity? They havefree moving electrons How does metal conduct heat? Atoms gain KE and pass this energy to neighboring atoms Explain why are non-metals not malleable? layers of atoms cannot slide over each other Explain why are non-metals weak(not strong)and brittle? Non-metlas have weak force of attraction between particles

Revision Chapter 3 (acids and bases) Name 3 strong acids 1) 2) Name 3 strong bases 3) Name the acid present in vinegar and nail polish 4) Name the acid present in vitamin C and lemon juice 5) Name the acid present in carbonated drinks 6) Name the acid present in your stomach 7) Name 2 indicators used to estimate the p. H value 8) Name the process of adding acids to bases 9) Name a substance that can be used to treat wasp sting? 10) Name a substance that can be used to treat bee st ing? 11) When acids are added to bases, does the p. H of the base increase or decrease? 12) What colour would universal indicator change to when a strong acid is added to a strong base? 13) Name 3 physical properties of acids 14) Name 3 physical properties of bases 15) Name 3 reactions of acids (chemical properties)

Revision Chapter 3 (acids and bases) Name 3 strong acids hydrochloric, sulfuric and nitric acid 1) 2) Name 3 strong bases detergent, bleach, sodium hydroxide 3) Name the acid present in vinegar and nail polish ethanoic acid and acetic acid 4) Name the acid present in vitamin C and lemon juice citric acid 5) Name the acid present in carbonated drinks carbonic acid 6) Name the acid present in your stomach hydrochloric acid 7) Name 2 indicators used to estimate the p. H value red cabbage, universal indicator 8) Name the process of adding acids to bases neutralisation 9) Name a substance that can be used to treat wasp sting? vinegar 10) Name a substance that can be used to treat bee sting? Toothpaste, sodium carbonate 11) When acids are added to bases, does the p. H of the base increase or decrease? decrease 12) What colour would universal indicator change to when a strong acid is added to a base? Blue red 13) Name 3 physical properties of acids sharp smell, stingy touch, sour 14) Name 3 physical properties of bases odorless, slippery and bitter 15) Name 3 reactions of acids (chemical properties) acid +metal/carbonate/hydroxide/oxide

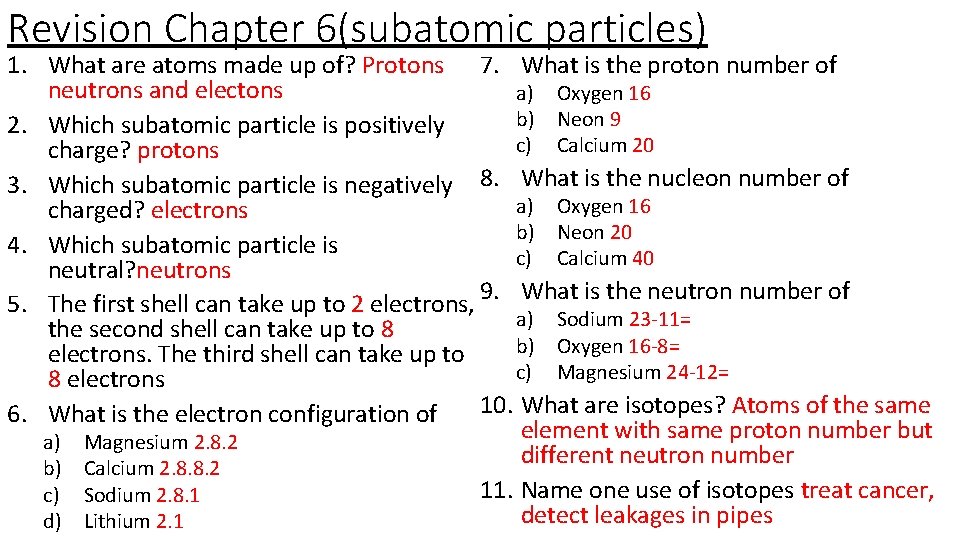
Revision Chapter 6(subatomic particles) 7. 1. What are atoms made up of? 2. Which subatomic particle is positively charge? 3. Which subatomic particle is 8. negatively charged? 4. Which subatomic particle is neutral? 5. The first shell can take up to ___ electrons, the second shell can take 9. up to ___ electrons. The third shell can take up to ___ electrons 6. What is the electron configuration of a) b) c) d) Magnesium Calcium Sodium Lithium What is the proton number of a) Oxygen b) Neon c) Calcium What is the nucleon number of a) Oxygen b) Neon c) Calcium What is the neutron number of a) Sodium b) Oxygen c) Magnesium 10. What are isotopes? 11. Name one use of isotopes

Revision Chapter 6(subatomic particles) 1. What are atoms made up of? Protons 7. What is the proton number of neutrons and electons a) Oxygen 16 b) Neon 9 2. Which subatomic particle is positively c) Calcium 20 charge? protons 3. Which subatomic particle is negatively 8. What is the nucleon number of a) Oxygen 16 charged? electrons b) Neon 20 4. Which subatomic particle is c) Calcium 40 neutral? neutrons 9. What is the neutron number of 5. The first shell can take up to 2 electrons, a) Sodium 23 -11= the second shell can take up to 8 b) Oxygen 16 -8= electrons. The third shell can take up to c) Magnesium 24 -12= 8 electrons 6. What is the electron configuration of 10. What are isotopes? Atoms of the same element with same proton number but a) Magnesium 2. 8. 2 different neutron number b) Calcium 2. 8. 8. 2 11. Name one use of isotopes treat cancer, c) Sodium 2. 8. 1 detect leakages in pipes d) Lithium 2. 1

Revision Chapter 6(Separating techniques and gas test) 1. Name 4 separation techniques. Exam (draw 1) 2. After filtration, what is the name of the insoluble substances in the filter paper? 3. After filtration, what is the name of the solution that passes through the filter paper? 4. Why is pencil line used instead of an ink line in chromatography 5. If the coloured ink does not move, is the coloured ink soluble or insoluble in the solvent? 6. What is the gas test for oxygen? 7. What is the gas test for carbon dioxide? 8. What is the gas test for hydrogen? 9. What is the name of the potential hazards below

Revision chapter 6(Separating techniques and gas test) 1. Name 4 separation techniques. Exam (draw 1) chromatography, evaporation, 2. 3. 4. 5. 6. 7. 8. 9. distillation, filtration After filtration, what is the name of the insoluble substances in the filter paper? Residue After filtration, what is the name of the solution that passes through the filter paper? filtrate Why is pencil line used instead of an ink line in chromatographypencil is not soluble in solvent If the coloured ink does not move, is the coloured ink soluble or insoluble in the solvent? insoluble What is the gas test for oxygen? relight a glowing wooden splinter What is the gas test for carbon dioxide? Turns limewater cloudy What is the gas test for hydrogen? burns lighted wooden splinter with a squeaky pop sound What is the name of the potential hazards below

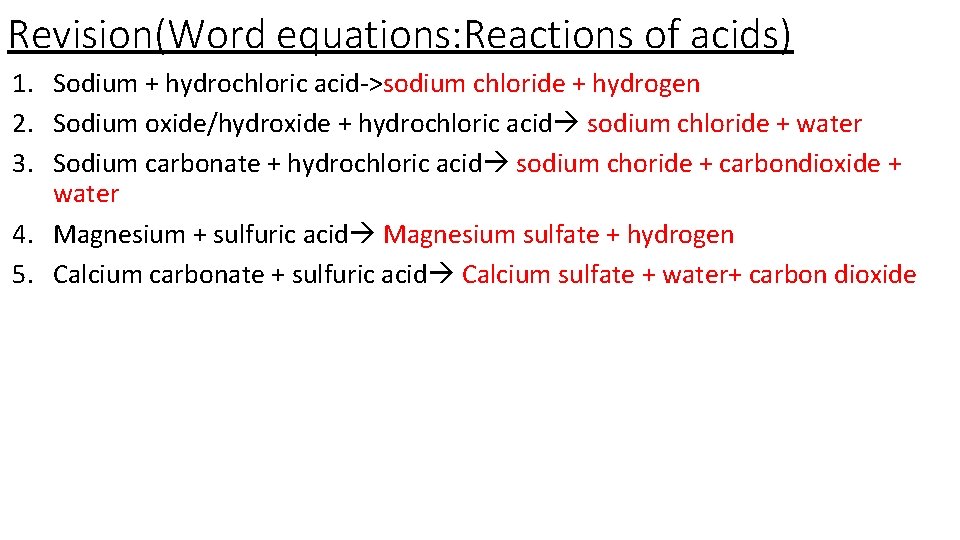
Revision(Word equations: Reactions of acids) 1. 2. 3. 4. 5. Sodium + hydrochloric acid Sodium oxide/hydroxide + hydrochloric acid Sodium carbonate + hydrochloric acid Magnesium + sulfuric acid Calcium carbonate + sulfuric acid

Revision(Word equations: Reactions of acids) 1. Sodium + hydrochloric acid->sodium chloride + hydrogen 2. Sodium oxide/hydroxide + hydrochloric acid sodium chloride + water 3. Sodium carbonate + hydrochloric acid sodium choride + carbondioxide + water 4. Magnesium + sulfuric acid Magnesium sulfate + hydrogen 5. Calcium carbonate + sulfuric acid Calcium sulfate + water+ carbon dioxide

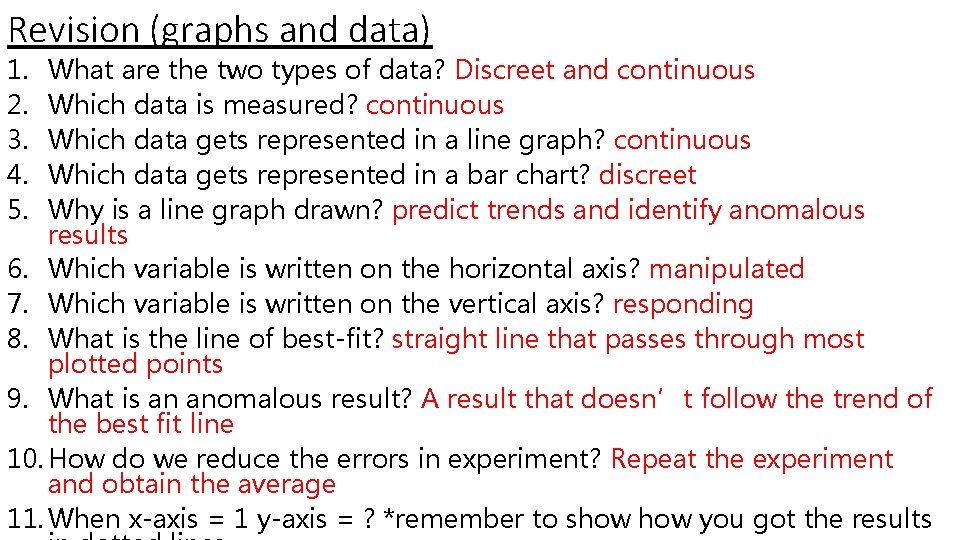
Revision (graphs and data) 1. What are the two types of data? 2. Which data is measured? 3. Which data gets represented in a line graph? 4. Which data gets represented in a bar chart? 5. Why is a line graph drawn? 6. Which variable is written on the horizontal axis? 7. Which variable is written on the vertical axis? 8. What is the line of best fit? 9. What is an anomalous result? *must know how to identify* 10. How do we reduce the errors in experiment? 11. When x-axis = 1 y-axis = ? *remember to show you got the results in dotted lines

Revision (graphs and data) 1. 2. 3. 4. 5. What are the two types of data? Discreet and continuous Which data is measured? continuous Which data gets represented in a line graph? continuous Which data gets represented in a bar chart? discreet Why is a line graph drawn? predict trends and identify anomalous results 6. Which variable is written on the horizontal axis? manipulated 7. Which variable is written on the vertical axis? responding 8. What is the line of best-fit? straight line that passes through most plotted points 9. What is an anomalous result? A result that doesn’t follow the trend of the best fit line 10. How do we reduce the errors in experiment? Repeat the experiment and obtain the average 11. When x-axis = 1 y-axis = ? *remember to show you got the results