Rest Energy and Rest Mass Elliott Fundamental Particles







- Slides: 13

Rest Energy and Rest Mass Elliott

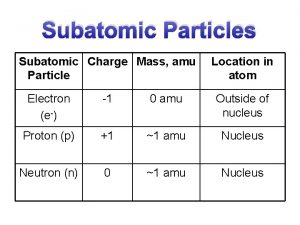
Fundamental Particles? • It used to be thought that protons, neutrons and electrons were the fundamental particles of matter, which could not be broken down into anything smaller. • However it has been found that nucleons are made up of smaller particles, so nucleons are now not fundamental.

Units The electron-volt is not a voltage, but a unit of energy. To make sense of quantities involved, we need to look at the units used in particle physics for mass and energy. The electron volt is defined as: Joules and kilograms are far too big and clumsy at the particle level. The amount of energy that a single electron has when it is accelerated by a potential difference of 1 volt. 1 e. V = 1. 6 × 10 -19 J

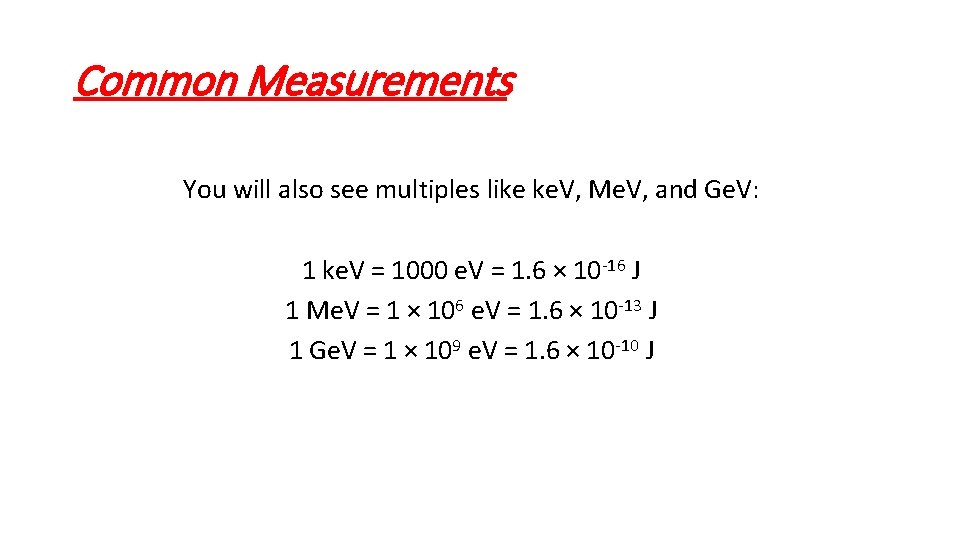
Common Measurements You will also see multiples like ke. V, Me. V, and Ge. V: 1 ke. V = 1000 e. V = 1. 6 × 10 -16 J 1 Me. V = 1 × 106 e. V = 1. 6 × 10 -13 J 1 Ge. V = 1 × 109 e. V = 1. 6 × 10 -10 J

Worked Example A photon has frequency of 1. 0 × 1018 Hz. What is its energy in J and e. V? Answer E = hf = 6. 63 × 10 -34 J s × 1018 Hz = 6. 63 × 10 -16 J. E = 6. 63 × 10 -16 J ÷ 1. 6 × 10 -19 J e. V-1 = 4. 14 ke. V

Conversion to Joules To convert electron volt (e. V) to Joule (J), multiply by 1. 60 × 10 -19 J e. V-1 To convert Joule (J) to electron volt (e. V) , divide by 1. 60 × 10 -19 J e. V-1.

Check Your Progress

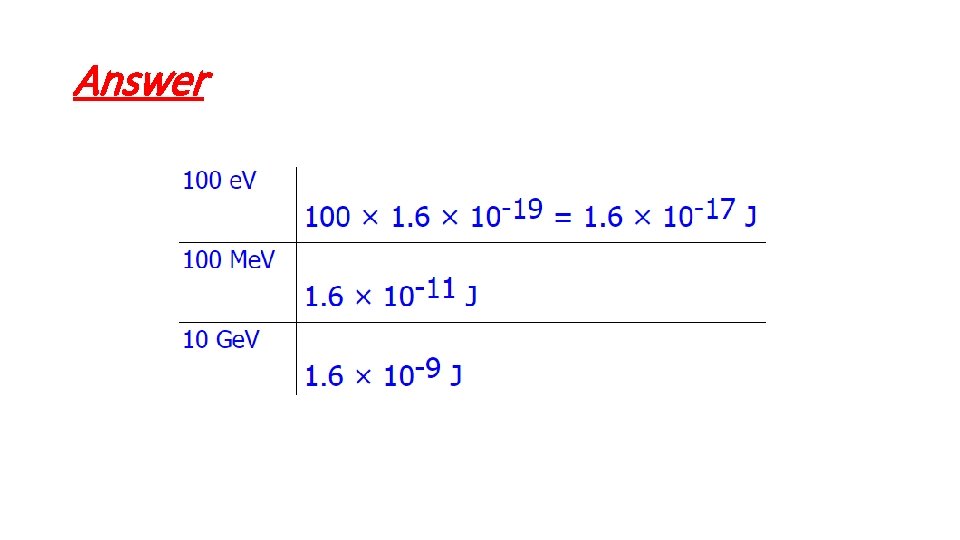
Answer

Atomic Mass Unit You will also see the atomic mass unit, u. The atomic mass unit is defined: Having exactly 1/12 th the mass of a carbon-12 atom.

Rest Mass and Rest Energy You will also come across an odd expression rest energy. At the subatomic level, mass and energy are one and the same thing. Mass can be turned into energy, and energy can be made into mass. They are linked by Einstein’s famous simple equation: E = mc 2

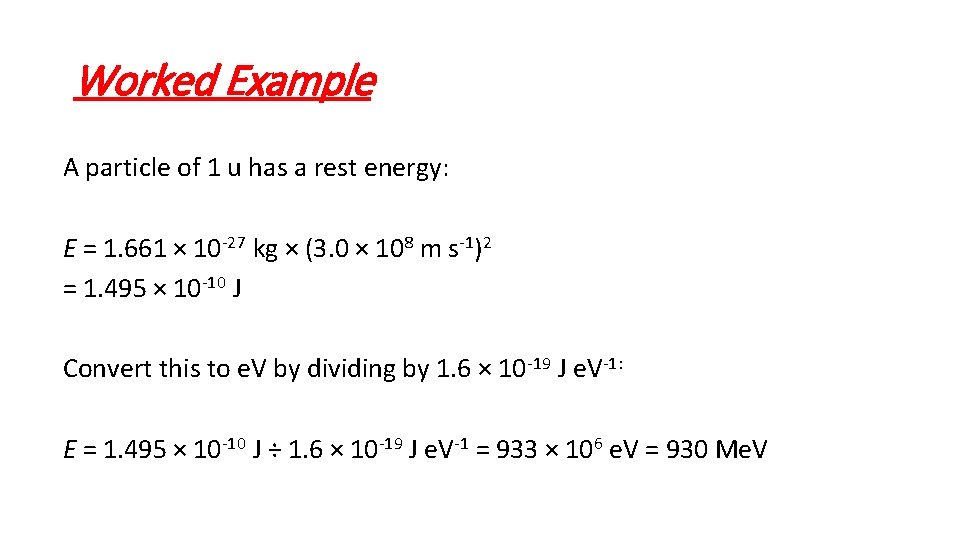
Worked Example A particle of 1 u has a rest energy: E = 1. 661 × 10 -27 kg × (3. 0 × 108 m s-1)2 = 1. 495 × 10 -10 J Convert this to e. V by dividing by 1. 6 × 10 -19 J e. V-1: E = 1. 495 × 10 -10 J ÷ 1. 6 × 10 -19 J e. V-1 = 933 × 106 e. V = 930 Me. V

Check Your Progress

Answers
 Microcosmos macrocosmos
Microcosmos macrocosmos Fundamental subatomic particles
Fundamental subatomic particles Mass of subatomic particles in amu
Mass of subatomic particles in amu Mass of subatomic particles
Mass of subatomic particles Mass of protons
Mass of protons Cingulum rests
Cingulum rests Rest seat
Rest seat Incisal rest
Incisal rest Heat transfer radiation
Heat transfer radiation Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Elliott erwitt biography
Elliott erwitt biography Ecole pierre elliott trudeau
Ecole pierre elliott trudeau Dana elliott
Dana elliott