Research Methods Leadership 389 Ethics in Research w





















- Slides: 22

Research Methods Leadership 389

Ethics in Research w As behavioral scientists, there are two domains of ethical responsibility: n n Truthfulness, integrity, etc. in the collection, interpretation and dissemination of data and theory. Humane treatment of participants

Integrity w This is a general issue in all sciences. It is the scientist’s responsibility to present the data and not alter, fabricate or suppress it. w When fraudulent data are published, it is eventually discovered because others try to replicate the data. w When integrity escapes a psychologist n n n Fabricated 4 published studies Banned from receiving federal funds for 5 years She resigned from her university in June, 2001

Nazi War Crimes w Sterilization Research w Pain/Disease & Performance w Trials at Nuremberg

Nazi War Crimes. Sterilization Research w Three experiments involving sterilization were in progress when World War II ended in 1945. n n n Dried plant juice was put into flour that was fed to the general population. This was supposed to sterilize women predominantly. Men stood at a counter to complete forms while being exposed, without their knowledge, to sterilizing doses of X-radiation. Intra-uterine injections of a silver nitrate solution were given to women, without their consent, during routine physical examinations

Nazi War Crimes Disease & Research w Between 1942 and 1943, about 729 people were subjected to “vaccine” experiments and 154 died. w In addition, other prisoners served as a "passage group. " -researchers would inject the virus into prisoners. When these people developed the acute illness, their blood was removed and injected into other prisoners.

The 10 Points of the Nuremberg Code 1. Participation must be voluntary, and subjects should have the capacity to give consent. Further, subjects should be fully informed of the purposes, nature, and duration of the experiment. 2. The research should yield results that are useful to society and that cannot be obtained in any other way. 3. The research should have a sound footing in animal research and be based on the natural history of the problem under study. 4. Steps should be taken in the research to avoid unnecessary physical or psychological harm to the subjects. 5. Research should not be conducted if there is reason to believe that death or disability will occur in the subjects.

The 10 Points of the Nuremberg Code 6. The risk involved in the research should be proportional to the benefits to be obtained. 7. Proper plans should be made and facilities provided to protect the subject from harm. 8. Research should be conducted by highly qualified scientists only. 9. The subject should have the freedom to withdraw at any time if he or she has reached the conclusion that continuing in the experiment is not possible. 10. The researcher must be prepared to discontinue the experiment if it becomes evident that continuing will be harmful to the subject.

Did the Nuremberg Code “suggestions” impact research practices? w Jewish Chronic Disease Hospital Study n n Study: The nature of the human transplant rejection process Injection of live cancer cells into patients who were hospitalized with various chronic debilitating diseases Researchers said that consent had been given orally, but was not documented. Further, patients were not told that they would receive cancer cells because, in the view of the investigators, this would frighten the patients unnecessarily.

Harvard Radiation Tests w From 1946 to 1956, 19 boys who thought they were participating in a science club were fed radioactive milk by researchers who wanted to learn about the digestive system. w The experiments were performed at the Fernald State School in Massachusetts. Researchers from Harvard University and MIT fed radioactive forms of iron and calcium to the boys, sometimes in their breakfast milk, to study the body's ability to digest minerals.

The Tuskegee syphilis study w 399 black men with syphilis and 201 men without syphilis, who served as the controls, were the subjects. w The men were recruited without informed consent. w Participants were misinformed and told that some of the procedures done in the interests of research (e. g. , spinal taps) were actually "special free treatment" and told that they had “bad blood. ” w Participants were prevented from obtaining treatments for the disease as they became available

The Tuskegee Syphilis Study The study continued until the first accounts of it appeared in the national press in 1972, at which time an ad hoc advisory panel was formed by the government to give advice on how to assure that such experiments would never again be conducted.

The Tuskegee Syphilis Study w The government continues to pay millions of dollars yearly to surviving subjects and the families of deceased subjects.


2 years later, National Laws Established, 1974

The Belmont Report w The primary task of the National Commission was to identify the ethical principles that would guide all research involving humans. The Belmont Report -- Ethical Principles and Guidelines for the Protection of Human Subjects was published in 1978. w The principles of The Belmont Report govern all research supported by the U. S. government today.

Current APA Standards 3 basic factors 1. Respect for Persons: This principle acknowledges the dignity and freedom of every person. It requires obtaining informed consent from research subjects (or their legally authorized representatives). 2. Beneficence: This principle requires that researchers maximize benefits and minimize harms associated with research. Research-related risks must be reasonable in light of expected benefits. 3. Justice: This principle requires equitable selection and recruitment and fair treatment of research subjects.

Ethical Treatment of Human Participants w Informed consent n Explain the procedures to be used any details that the participant needs to know to decide whether or not to participate. Extra steps are needed here for minors and others who might feel coerced into participating w Individual freedom to withdraw n Individuals have the right not to participate and may withdraw at any time even if they do consent to participate.

Ethical Treatment of Human Participants w Use of deception n Are there aspects of the study that are concealed from the participant? Is this necessary for the conduct of the study? Does this increase the risk? w Protection from physical/psychological harm n Researchers cannot knowingly put participants in dangerous situations w Confidentiality of data n Data and the identity of participants are confidential and not to be revealed.

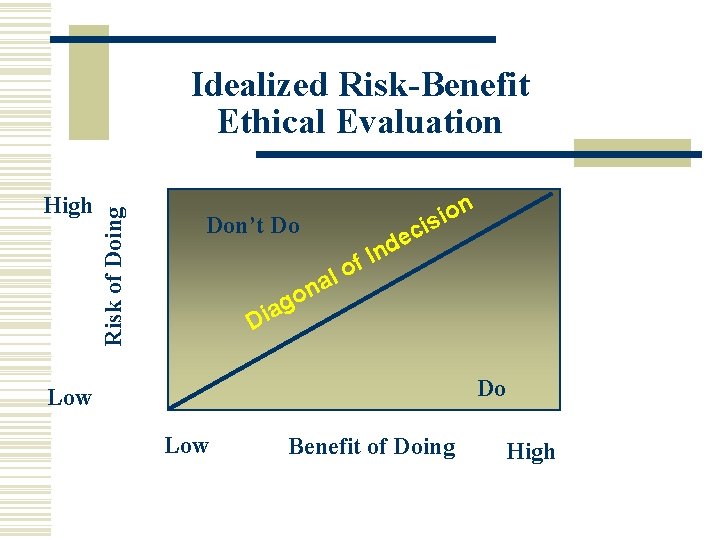
Ethical Treatment of Human Participants w Evaluate risk/benefit ratio n Risk/Benefit ratio. Are there psychological and/or physical risks to the participant from the study? What are the benefits to the participant or society from the research? w Debriefing n Any aspects not explained in the initial informed consent should be explained in debriefing. The use of deception should be explained.

High Risk of Doing Idealized Risk-Benefit Ethical Evaluation Don’t Do n o i is c de In f lo a on g a i D Do Low Benefit of Doing High

Ethics Activity w Textbook pages 64 -65: n Group 1: Activity Question 1 n Group 2: Activity Question 2 n Group 3: Activity Question 3 n Group 4: Activity Question 5
 Gi bill debt management
Gi bill debt management 0 phẩy 856 m bằng bao nhiêu cm
0 phẩy 856 m bằng bao nhiêu cm 389 directory
389 directory A-wax pattern recognition
A-wax pattern recognition Tok exhibition sample
Tok exhibition sample Leadership methods
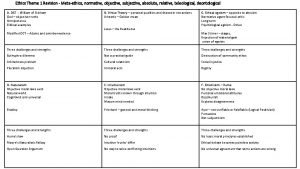
Leadership methods Descriptive ethics vs normative ethics
Descriptive ethics vs normative ethics Briefly summarise
Briefly summarise Micro and macro ethics
Micro and macro ethics 6075 meaning
6075 meaning Metaethics vs normative ethics
Metaethics vs normative ethics Descriptive ethics vs normative ethics
Descriptive ethics vs normative ethics Beneficence examples
Beneficence examples Is/ought distinction
Is/ought distinction Metaethics vs normative ethics
Metaethics vs normative ethics Deontological ethics
Deontological ethics Teleological ethics vs deontological ethics
Teleological ethics vs deontological ethics Transactional vs transformational leadership
Transactional vs transformational leadership Situational leadership vs adaptive leadership
Situational leadership vs adaptive leadership Capable but cautious performer
Capable but cautious performer Alan bryman
Alan bryman Research method notes
Research method notes Method of social psychology
Method of social psychology