Research Ethics Management Online REMO Getting Started REMO











- Slides: 13

Research Ethics Management Online (REMO) Getting Started

REMO Website • https: //remo. ualberta. ca – link from U of A and Research Ethics Office (www. reo. ualberta. ca) websites • Browsers: Google Chrome is preferred browser, Safari is not compatible with REMO • The first time you use REMO, disengage your pop-up blocker and make REMO a trusted site

Getting Started • Faculty members & Clinicians • REMO investigator role auto-generated – First time log in • Researcher must identify department for approval routing • Study coordinators, students & outside applicants (AHS/Covenant Health, guest applicants) • Log into REMO using CCID and password • Request Additional Role (https: //www. youtube. com/watch? v=a. Xx 4 osy. Rsr. E)

Students • Must select their department as “Student” from the department list when they complete registration in REMO • Must answer “Yes” to the question “Are you a Student/Trainee Researcher? ” • Post docs, Medical Residents, Ph. D & Masters students are all considered students when completing an ethics application as part of their program

Student & Trainee Research REB 1&2 • Student is the PI • After submission, study routes to named supervisor, who must approve prior to being forwarded to the REB • PI & supervisor both receive REMO email notifications HREB (Health and Biomedical) • Student, Post Doc, Resident is Co-I, PI is their “supervisor” • Co-I prepares application, PI must submit

REB 1&2 Work Flow Submit Ethics Application Changes Requested Faculty Supervisor Required for Student Investigators REB Coordinator Full Board Review Delegated Reviewer

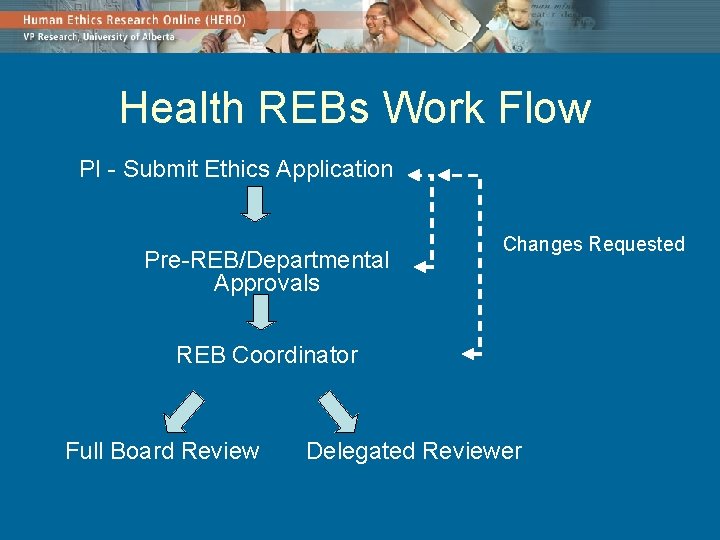
Health REBs Work Flow PI - Submit Ethics Application Pre-REB/Departmental Approvals Changes Requested REB Coordinator Full Board Review Delegated Reviewer

Study Access • Who can access a study? – When preparing an application, the PI, study coordinator and all co-investigators can read and edit the application. ONLY the PI can submit the study the first time – Post submission, the PI, SC and Co-I’s can respond to requests for changes – To give view only access to someone you can add them to the Guest List for the study, all REMO users are available in the list – To add someone to receive all REMO notifications about the study, add them to the Study Email List

Email Notifications • • Initial submission Change requests from reviewers / supervisors REB decision Renewal reminders – except when an amendment is pending • Approval • Supervisors receive notifications • Edit Study Email List to add other REMO users to email notifications • Notifications automatically go to the PI, and Study Coordinators, the Co-Investigators must be added to the email list to receive all notices. Important: REMO sends only to @ualberta. ca email addresses. You get this once you get a CCID.

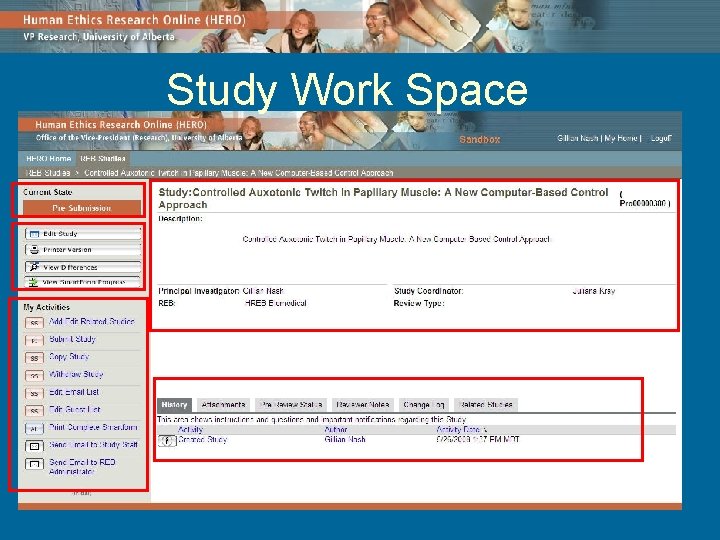
Study Work Space

Approval • Email notification telling you that your study has been approved –this notification is NOT the approval letter • Where to look for the approval letter • How to save the approval letter • How to print the approval letter

Post Approval Activity • Approval for 364 days, if study still active must renew. • System will prompt with emails at 30, 15 and 10 days • ANY changes to approved research require REB review BEFORE they are implemented.

REMO Resources • Research Ethics Office Website – www. reo. ualberta. ca for resources and contact information & templates • VIDEO Tutorials: http: //www. reo. ualberta. ca/Education. Training-User-Support/REMO-Support/Video. Tutorials. aspx People to call • • • REMO Help - Kathy Strawson 780 -492 -0459 REB 1 & 2 – Melanie Pankratow 780 -492 -7550 REB 3 HREB Health Panel - Charmaine Kabatoff 780 -492 -0302 REB 4 HREB Biomedical Panel - Patricia Lo 780 -492 -9724, REB 4 HREB Biomedical Panel – Ann Moore 780 -492 -6459 Research Ethics Officer – Kim Kordov 780 -492 -2615