RAYAT SHIKSHAN SANSTHAS NANDRE VIDYALAYA NANDRE v STD10
















- Slides: 22


RAYAT SHIKSHAN SANSTHA’S NANDRE VIDYALAYA NANDRE

v. STD-10 `th v. Subject-Science & technology v. Content-Chapter 8 Understanding Metals and Non metals Name - Shri. Rajoba A. B.

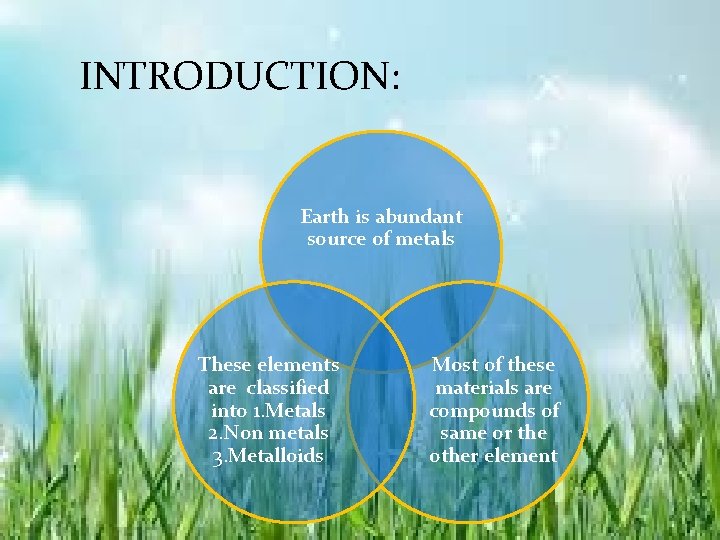
INTRODUCTION: Earth is abundant source of metals These elements are classified into 1. Metals 2. Non metals 3. Metalloids Most of these materials are compounds of same or the other element

List of sub units 8. 1 8. 2 8. 3 8. 4 Physical properties of metals Physical properties of non metals Chemical properties of metals Occurrence of metals : 1. Activity series & related metallurgy 2. Exraction of metals of high reactivity 3. Extraction of metals of medium reactivity 4. Extraction of metals of low reactivity 8. 5 Corrosion of metals List of sub units

New subunits : 0 no. Sub-units New sub-units 8. 1 Physical properties of Metals 1. Hardness 2. Melting point & boiling point 8. 2 Physical properties of Non-Metals Nil 8. 3 Chemical properties of metals 8. 4 Occurrence of metals 8. 5 Corrosion of metals 1. ) Reaction of metals with oxygen 2. ) Reaction of metals with H 2 O 3. )Reaction of metals with Acids 4. )Reaction of metals with Solutions of other metals salts 5. ) Reaction of metals with non metals 1. Activity series & related metallurgy 2. Exraction of metals of high reactivity 3. Extraction of metals of medium reactivity 4. Extraction of metals of low reactivity 1. Prevention of corrosions (Galvanizing, Electroplating , Tinning, Anodising. & Alloying)

Explanation of new sub-units 8. 3. 1 Reaction of metal with oxygen Metal +Oxygen ---------- > Oxides of metals Reactivity differs for different metal 1. Most Reactive metals( Na , Mg) 4 Na + O 2 2 Na 2 O 2 Mg + O 2 2 Mg. O 2. Medium reactive metals(Zn , Fe , Pb ) Zinc burn in air : 2 Zn + O 2 2 Zn. O Iron does not burn even on strong heating but iron filling vigorously when sprinkled in the flame of burner 3 Fe + 2 O 2 Fe 3 O 4 3. Less reaction metal(Cu , Hg , Au) Cu does not burn but on heating it Coates black colour layer of copper oxide 2 Cu + O 2 2 Cu. O

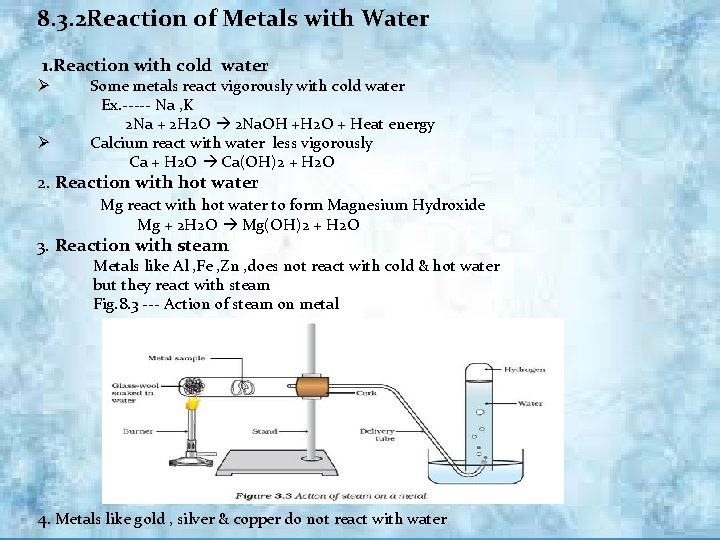
8. 3. 2 Reaction of Metals with Water 1. Reaction with cold water Ø Ø Some metals react vigorously with cold water Ex. ----- Na , K 2 Na + 2 H 2 O 2 Na. OH +H 2 O + Heat energy Calcium react with water less vigorously Ca + H 2 O Ca(OH)2 + H 2 O 2. Reaction with hot water Mg react with hot water to form Magnesium Hydroxide Mg + 2 H 2 O Mg(OH)2 + H 2 O 3. Reaction with steam Metals like Al , Fe , Zn , does not react with cold & hot water but they react with steam Fig. 8. 3 --- Action of steam on metal 4. Metals like gold , silver & copper do not react with water

8. 3. 3 Reaction metals with Acids General Reaction : Metal + Acid -----) Salt+ Hydrogen 1. Reaction with dil. HCl To form metal chloride & hydrogen gas In these case rate of formation of bubble was fastest in the order Mg>Al>Zn>Fe Mg + 2 HCl Mg. Cl 2 + H 2 2. No bubbles are seen in case of copper i. e. Cu does not react with dil. HCl 3. Reaction with Sulphuric Acid To form metal sulphate & hydrogen gas Zn + H 2 SO 4 Zn. SO 4 + H 2 O 4. Reaction with Nitric Acid In these reaction hydrogen gas is not evolved because , metal is strong oxidizing agent It oxides hydrogen to water

Aquaregia (Aamlaraja ) 1. It highly corrosive as well as fuming liquid 2. Is one of few Reagents that is able to dissolved gold & platinum It is mixture of con. HCl acid & con. Nitric acid in the ratio of 3: 1 Aquaregia = Con. HCl + Con. HNO 3

8. 3. 4 Reaction of metals with solution of other metal salts Fig. 8. 4 Activity : 1. Take a clean Cu & iron nail 2. Put Cu wire in solution of Mg. Cl 2 Observation : 1. The iron nail get coated with reddish brown color of copper & blue color of Cu. SO 4 Conclusion : A more reactive , metal can displace less active metal from its compound in solution Metal A + Salt solution B------- Salt solution A + Metal B Q: Why color of solution of Cu. SO 4 become Faint?

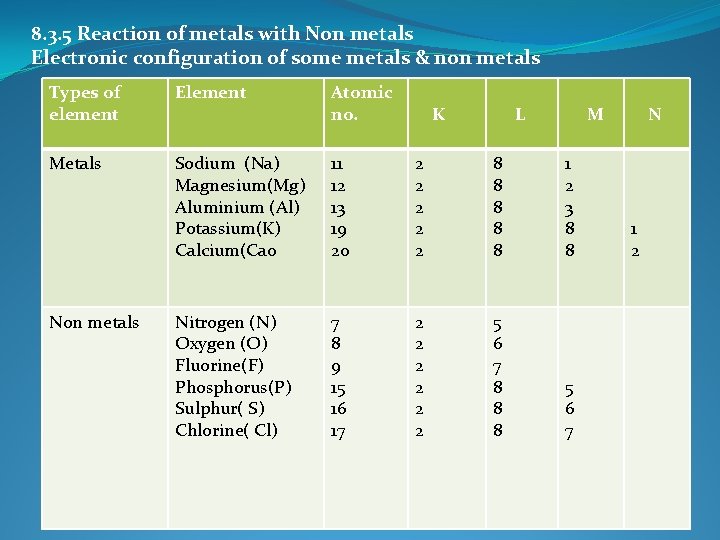
8. 3. 5 Reaction of metals with Non metals Electronic configuration of some metals & non metals Types of element Element Atomic no. Metals Sodium (Na) Magnesium(Mg) Aluminium (Al) Potassium(K) Calcium(Ca 0 11 12 13 19 20 2 2 2 8 8 8 1 2 3 8 8 Nitrogen (N) Oxygen (O) Fluorine(F) Phosphorus(P) Sulphur( S) Chlorine( Cl) 7 8 9 15 16 17 2 2 2 5 6 7 8 8 8 5 6 7 Non metals K L M N 1 2

Reaction of metals with non metals In this case , The transfer of electrons from metal to non metal & to form electro-covalent bond or Ionic bond And resulting formation of ionic compounds Ex: Formation of Na. Cl Sodium + Chlorine---- sodium chloride (Metal ) (non metal) (Compound) (2, 8, 1) (2, 8, 7) (2, 8, 8) 1. Na & Cl ion being opposite charged attract to each other And held by strong electrostatic force of attraction 2. Resulting formation of ionic bond or electro covalent bond & to form Na. Cl Fig: 8. 6 Formation of sodium chloride

IONIC COMPOUNDS IONIC BOND: “The bonds which are formed by such a give and take of electrons” are called as ionic bonds. IONIC COMPOUNDS : “The compounds formed by transfer of electrons from metal to non-metal ” are called ionic compounds. PROPERTIES OF IONIC COMPOUNDS : Give some activity : Ø Take sample and observe physical state.

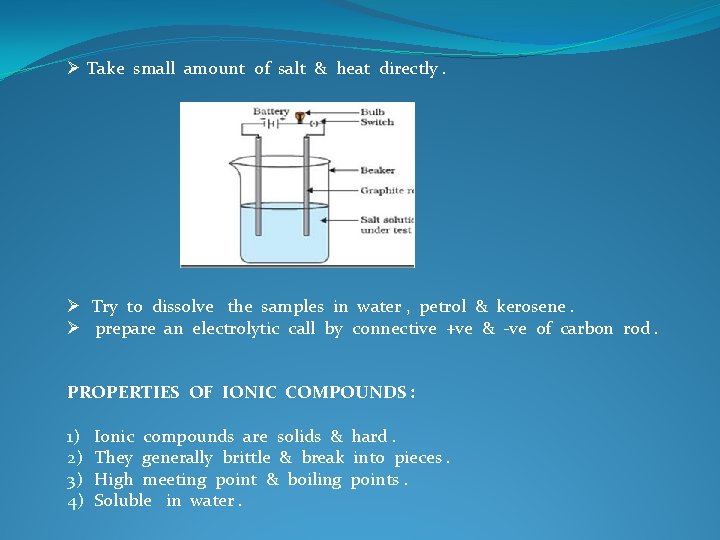
Ø Take small amount of salt & heat directly. Ø Try to dissolve the samples in water , petrol & kerosene. Ø prepare an electrolytic call by connective +ve & -ve of carbon rod. PROPERTIES OF IONIC COMPOUNDS : 1) 2) 3) 4) Ionic compounds are solids & hard. They generally brittle & break into pieces. High meeting point & boiling points. Soluble in water.

OCCURRENCE OF METALS Ø Metals occurs in nature in free as well as in combined states Ø Some of unreactive metal generally found in free or native state. MINERALS : “The naturally occurring compounds of metals along with impurities” ORES : “The minerals from which metal are extracted profitably & conveniently” GANGUE : They contain some impurities. METALLURGY : The process used for extraction of metals in their pure form their ores.

REACTIVITY SERIES & RELATED METALLRGY Definition : “ The arrangement of metals in the decreasing order of the reactivity in the form of series” is called “series of metals. k Na Ca Mg Al Zn Fe Pb Cu Hg Ag Au High reactive Medium reactive Less reactive On the basis of reactivity metals have 3 categories : Ø Metals of high reactivity. Ø Metals of Medium reactivity. Ø Metals of Less reactivity.

EXTRACTION OF METALS OF HIGH REACTIVITY 1) For example : - Na , k , Ca , Al etc. 2) These metals are obtained by electrolytic reduction. Extraction of ALUMINIUM : Two steps : - 1) Concentration of ore ( BARE`s PROCESS) i. e conversion of Bauxite into Alumina. 2) Electrolytic reduction of pure “ALUMINA” Fig. 8. 9 Extraction of aluminium.

EXTRACTION OF METALS MEDIUM REACTIVITY Ø Medium reactive metals -- Fe , Zn , Pb , Cu. Ø These are present as sulphades or carbonates. Ø ROASTING : “The sulphide are converted into oxides by strongly heating in excess of air” this process is called roasting. Ø CALCINATION : “The carbonates are changed into oxide by strongly heating in limited air ” this process is called as calcination. For ex. 2 Zns + 3 O 2 2 Zno + 2 So 2 Zn. Co 3 Zno + Co 2 Ø REDUCTION : Zinc oxides reduced to Zinc by using suitable reducing agents such as carbon. Ø Some times Al , Ca , Na highly reactive metals are also used as reducing agents.

THERMITE PROCESS : In this reaction , iron oxides react with Al to give iron & Al 2 O 3 & evolve lots of heat. Ø These displacement reaction are highly exothermic. Amount of heat is so large that the metal are produced in molten state. Ø In fact , the combination of iron oxide with Al is used for making RAILWAY TRACKS and MEACHINE parts.

EXTRACTION OF METALS OF LOW REACTIVITY Ø Lower reactive metal : -- Gold , Silver , Copper Ø They are often found in free state. Ex. Au , Cu Ø But Cu found in Cu 2 S & these are obtained by heating ORE Ø Similarly Hgs is ore of mercury. CORROSION OF METALS DEFINATION : - “It is degradation of materials due to reaction with its environments”. OCCURRENCE : Ø Old iron grills Ø Not cleaned copper vessels Ø Silver ornament ( which exposed in air for long time)

PREVENTION OF CORROSION Ø Corrosion of metals can be prevented by avoiding contact between metal and air. Some methods : 1) 2) Corrosion prevented if coated. Coating of metals with paint / oil / grease or varnish. Ø Some methods by which metals can be coated with non-corrosive metals. 1) 2) 3) 4) 5) Galvanizing Tinning Electroplating Alloying Anodizing Ex. Brass / Bronze / Stainless steel
 Rayat shikshan sanstha sadhana vidyalaya hadapsar
Rayat shikshan sanstha sadhana vidyalaya hadapsar Sadhana vidyalaya hadapsar
Sadhana vidyalaya hadapsar Rayat shikshan sanstha sadhana vidyalaya hadapsar
Rayat shikshan sanstha sadhana vidyalaya hadapsar Rayat shikshan sanstha motto
Rayat shikshan sanstha motto Rayat shikshan sanstha loni
Rayat shikshan sanstha loni Concurant
Concurant Education through self help is our motto
Education through self help is our motto Rayat rose
Rayat rose Rayat shikshan sanstha kamothe
Rayat shikshan sanstha kamothe उद्दीपकाचे
उद्दीपकाचे Kendriya vidyalaya bhu
Kendriya vidyalaya bhu Swami ramanand vidyalaya ramanandnagar
Swami ramanand vidyalaya ramanandnagar Maharaja sawai man singh vidyalaya
Maharaja sawai man singh vidyalaya Chhatrapati shivaji vidyalaya
Chhatrapati shivaji vidyalaya Kendriya vidyalaya sangathan bangalore
Kendriya vidyalaya sangathan bangalore Swami ramanand vidyalaya ramanandnagar
Swami ramanand vidyalaya ramanandnagar Objectives of kasturba gandhi balika vidyalaya
Objectives of kasturba gandhi balika vidyalaya Kvs jagdalpur
Kvs jagdalpur Kendriya vidyalaya lesson plan
Kendriya vidyalaya lesson plan Swach vidyalaya abhiyan
Swach vidyalaya abhiyan Sadhana kanya vidyalaya hadapsar
Sadhana kanya vidyalaya hadapsar Sant tukaram vidyalaya dehu
Sant tukaram vidyalaya dehu Shri bhairavdev vidyalaya
Shri bhairavdev vidyalaya