Radiopharmaceutical Production Personnel STOP Staffing Requirements for an





- Slides: 9

Radiopharmaceutical Production Personnel STOP

Staffing Requirements for an FDG facility • • • The facility for production of PET Contents radioisotopes and • Introduction radiopharmaceuticals/radiotracers will require staff representing a wide range of • Production Staff qualifications. • Support Staff The number as well as the qualification • Conclusions level of personnel that will be needed in order to maintain smooth operation of the • Additional Resources facility will be determined by the size and scope of the facility. In general, the staff should have the formal education, training and experience that are relevant to the assigned tasks. In a small facility, a single person may take on several of the jobs. More about key personnel and job responsibilities can be found by following the arrow. More Job Responsibilities STOP

Radiopharmaceutical Production Introduction • Personnel Contents Introduction Production Staff Support Staff Conclusions • Additional Resources • STOP The number of people working at a facility, their qualification and experience are a very important, if not the most important issue, for the efficient running of a PET facility. Many times, while designing a PET facility, It may be difficult to find the right people. It is very important that the people who will be responsible for the day to day operations of the facility be identified early and have training for their area of responsibilities. The technical supervisors and scientists should possess sufficient experience in their respective fields to guide and train the junior technical staff. The facility management should ensure that ample continuing education opportunities are provided for staff training to maintain and enhance performance. The functions involved in the workflow process are listed in the following slides and are divided into the production staff and the support staff. It must be emphasized that the staff are qualified personnel and with specialized training.

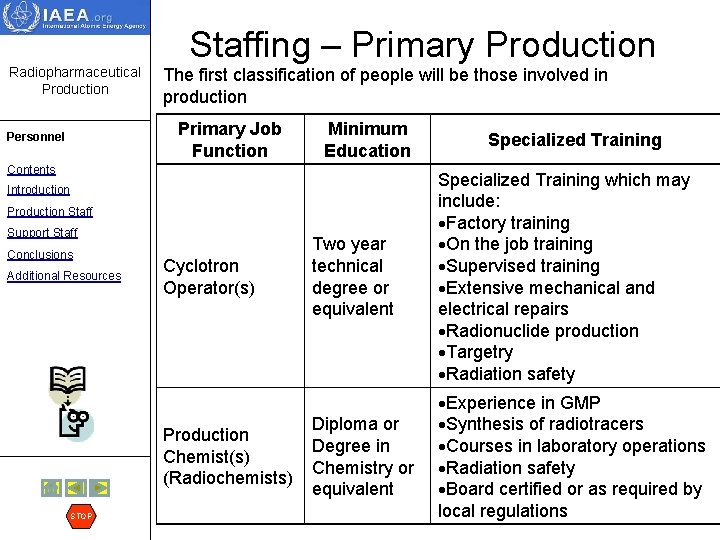
Staffing – Primary Production Radiopharmaceutical Production The first classification of people will be those involved in production Primary Job Function Personnel Minimum Education Contents Two year technical degree or equivalent Specialized Training which may include: Factory training On the job training Supervised training Extensive mechanical and electrical repairs Radionuclide production Targetry Radiation safety Diploma or Degree in Chemistry or equivalent Experience in GMP Synthesis of radiotracers Courses in laboratory operations Radiation safety Board certified or as required by local regulations Introduction Production Staff Support Staff Conclusions Additional Resources Cyclotron Operator(s) Production Chemist(s) (Radiochemists) STOP Specialized Training

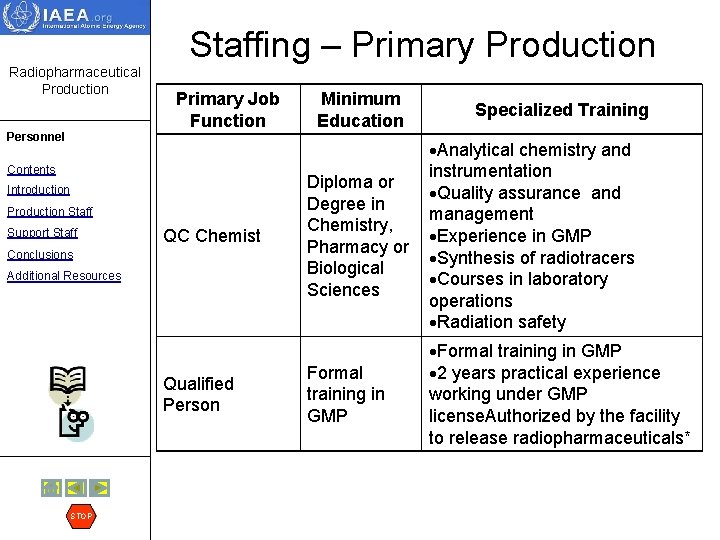
Staffing – Primary Production Radiopharmaceutical Production Personnel Primary Job Function Contents Introduction Production Staff Support Staff QC Chemist Conclusions Additional Resources Qualified Person STOP Minimum Education Specialized Training Diploma or Degree in Chemistry, Pharmacy or Biological Sciences Analytical chemistry and instrumentation Quality assurance and management Experience in GMP Synthesis of radiotracers Courses in laboratory operations Radiation safety Formal training in GMP 2 years practical experience working under GMP license. Authorized by the facility to release radiopharmaceuticals*

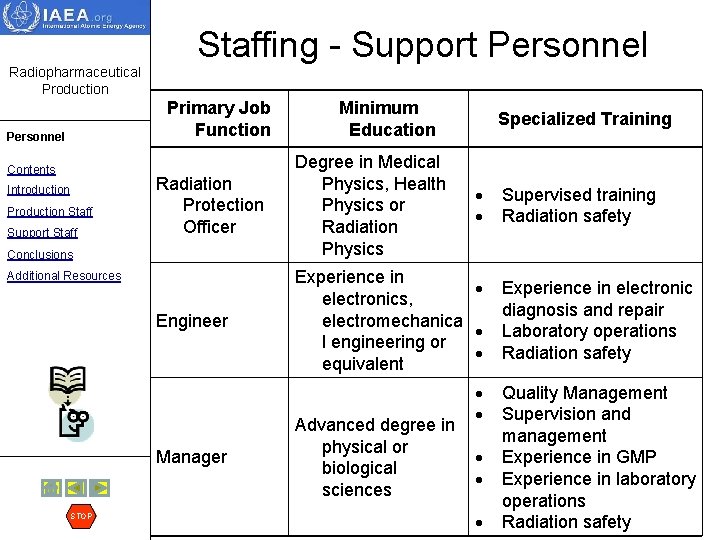
Staffing - Support Personnel Radiopharmaceutical Production Primary Job Function Personnel Contents Introduction Production Staff Support Staff Specialized Training Radiation Protection Officer Degree in Medical Physics, Health Physics or Radiation Physics Engineer Experience in electronics, electromechanica l engineering or equivalent Conclusions Additional Resources Manager STOP Minimum Education Advanced degree in physical or biological sciences Supervised training Radiation safety Experience in electronic diagnosis and repair Laboratory operations Radiation safety Quality Management Supervision and management Experience in GMP Experience in laboratory operations Radiation safety

Conclusions Radiopharmaceutical Production • Personnel • Contents Introduction Production Staff Support Staff Conclusions Additional Resources STOP • The size and scope of the facility, in general, will determine the number as well as the qualification level of personnel that will be needed to maintain efficient operation. The staff should have the formal education, training and experience that are relevant to the assigned tasks. While most of these employees would be required to be regular employees, some may be contracted from outside sources (e. g. , radiation protection officer and pharmacist).

Additional Resources Radiopharmaceutical Production • Cyclotron Produced Radionuclides: Production and Quality Control of FDG [Link Under Construction] • Cyclotron Produced Radionuclides: Guidelines for Setting Up a Facility TRS 471. • IAEA Human Health Series no. 11 Planning a PET Center Personnel Contents Introduction Production Staff Support Staff Conclusions Additional Resources STOP

Return to Main Menu STOP