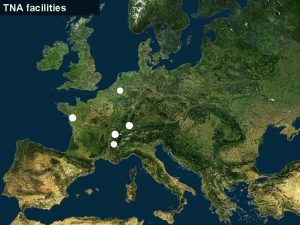
Radiopharmaceutical Production Cyclotron Facilities and FDG Radiopharmaceutical Production











- Slides: 22

Radiopharmaceutical Production Cyclotron Facilities and FDG Radiopharmaceutical Production Click here to proceed to the Introduction

Forward • • • The application of radionuclides in medicine has undergone significant growth in the last decade and one of the major factors contributing to this increased growth is the availability of a large number of cyclotrons exclusively dedicated to this purpose. Many of these cyclotrons are dedicated to the production of PET isotopes and more specifically, fluorine 18 for the production of 18 F-FDG (fluorodeoxyglucose). Grateful acknowledgement is given to the IAEA for sponsoring this project and to Brookhaven National Laboratory for support the electronic distribution of this material. Comments and suggestions may be sent to either – David Schlyer schlyer@bnl. gov or – Mohammad Haji-Saeid M. Haji-Saeid@iaea. org

Web Information Presentation This series of presentations is intended to be a readily accessible platform with background material and practical information on operating a radiopharmaceutical production facility with an emphasis on the production of FDG. The material was assembled by a group of people who are involved in PET radiopharmaceutical production. This tutorial describes processes and procedures that may be used in the Click here or on the forward arrow to proceed with the Explanation and Navigation preparation of PET radiopharmaceuticals. Other processes and procedures that are not described in this tutorial may also Click here to proceed directly to the Overview (not recommended for first time visitors) be acceptable Overview

Philosophical Organizational Structure This series of presentations is intended to be a readily accessible platform with background material and practical information on operating an FDG production facility, As an example: the operation of the cyclotron at different levels Basics Level 1 What is a cyclotron? Few Details Level 2 More Details Level 3 What are the major components of the cyclotron? How do the components work together and what is the basic theory? Complete (Not in these presentations) Level 4 What is theory and all the parameters behind the operation of each component?

Introduction This informational module is designed to give you an introduction to the production of radiopharmaceuticals. It is a self guided tour and you can learn about any specific topic by clicking on the pictures in the overview or by navigating to a specific presentation. You can navigate within a presentation using the navigation buttons shown on the right side of this slide. Click on the stop button to end the presentation you are viewing and return to the previous presentation. Hitting the stop button repeatedly will bring you back to this presentation. STOP Clicking on the blue arrows in each section will take you to a new presentation to learn more about a specific topic. More schlyer@bnl. gov Go on to the next slide Home button takes you to the table of contents slide Go to the next slide Go to the previous slide Go to the next section The back section button takes you to the beginning of the section

Radiopharmaceutical Production Cyclotron Operations FDG Production Cyclotron Targetry Radionuclide Transfer GMP Quality Control Product Release Dispensing - shipping Quality Assurance Click on a picture or box to learn more Production Environment Equipment Requirements Radiation Protection Validation Personnel Send comments to schlyer@bnl. gov

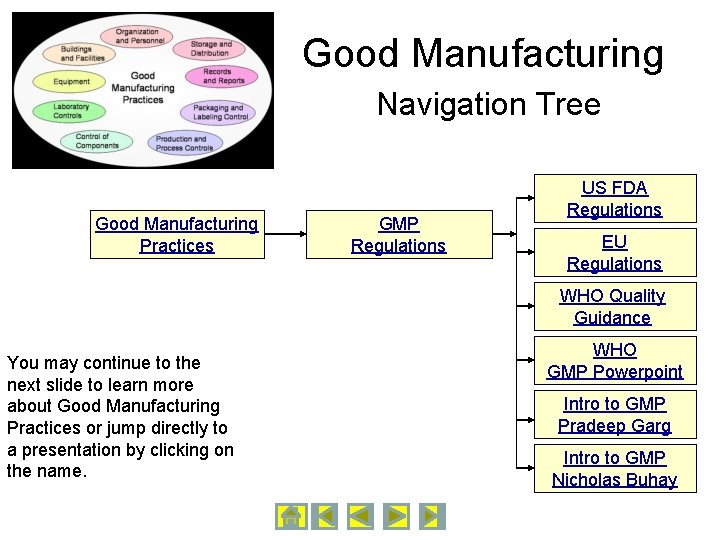
Good Manufacturing Navigation Tree Good Manufacturing Practices GMP Regulations US FDA Regulations EU Regulations WHO Quality Guidance You may continue to the next slide to learn more about Good Manufacturing Practices or jump directly to a presentation by clicking on the name. WHO GMP Powerpoint Intro to GMP Pradeep Garg Intro to GMP Nicholas Buhay

Good Manufacturing Practices • The purpose Of GMP is to assure the identity, strength, quality, and purity of drug products. This is accomplished by requiring that manufacturers of pharmaceuticals adequately control manufacturing operations. This includes establishing strong quality management systems, obtaining appropriate quality raw materials, establishing robust operating procedures, detecting and investigating product quality deviations, and maintaining reliable testing laboratories. This formal system of controls at a radiopharmaceutical production facility, if adequately put into practice, helps to prevent instances of contamination, mix-ups, deviations, failures, and errors. This assures that drug products meet their quality standards. GMP can be summarized as the necessary level of control over the operation and administration of facilities, methods and processes which will lead to the manufacture of radiopharmaceutical products of consistently high quality (to assure that the products meet the requirements as to safety and effectiveness which the product purports). More GMP More about GMP can be found here. Regulations

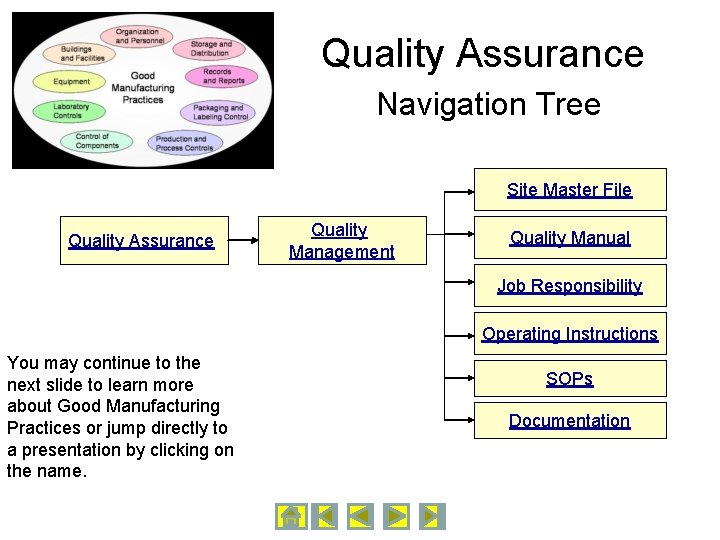
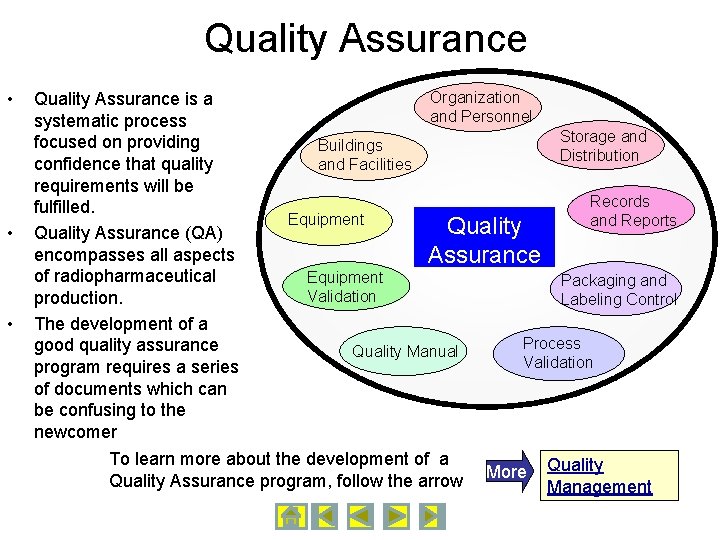
Quality Assurance Navigation Tree Site Master File Quality Assurance Quality Management Quality Manual Job Responsibility Operating Instructions You may continue to the next slide to learn more about Good Manufacturing Practices or jump directly to a presentation by clicking on the name. SOPs Documentation

Quality Assurance • • • Quality Assurance is a systematic process focused on providing confidence that quality requirements will be fulfilled. Quality Assurance (QA) encompasses all aspects of radiopharmaceutical production. The development of a good quality assurance program requires a series of documents which can be confusing to the newcomer Organization and Personnel Storage and Distribution Buildings and Facilities Equipment Quality Assurance Equipment Validation Quality Manual To learn more about the development of a Quality Assurance program, follow the arrow Records and Reports Packaging and Labeling Control Process Validation More Quality Management

Personnel Navigation Tree Personnel and Quality Assurance Personnel You may continue to the next slide to learn more about Personnel and Quality Assurance or jump directly to a presentation by clicking on the name. Job Responsibilities

Personnel • The facility for production of PET radioisotopes and radiopharmaceuticals/radiotracers will require a staff representing a wide range of job titles and training requirements. The staff should have the formal education, training and experience necessary to carry out the assigned tasks. The job functions include both production personnel and quality control personnel as well as some support functions Personnel More • Quality Assurance is a way of thinking to ensure that the product being produced is effective and safe for human use. Quality Assurance includes the validation of equipment, the validation of the process, and the documentation. More Job Responsibilities

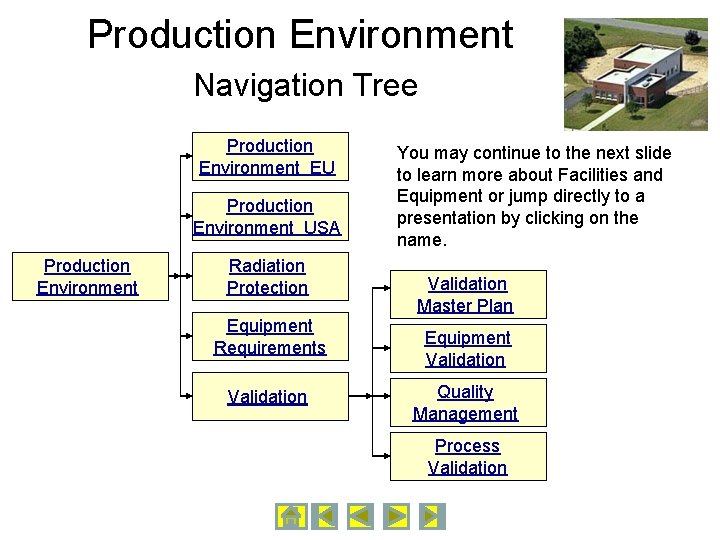
Production Environment Navigation Tree Production Environment EU Production Environment USA Production Environment Radiation Protection Equipment Requirements Validation You may continue to the next slide to learn more about Facilities and Equipment or jump directly to a presentation by clicking on the name. Validation Master Plan Equipment Validation Quality Management Process Validation

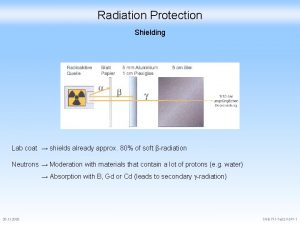
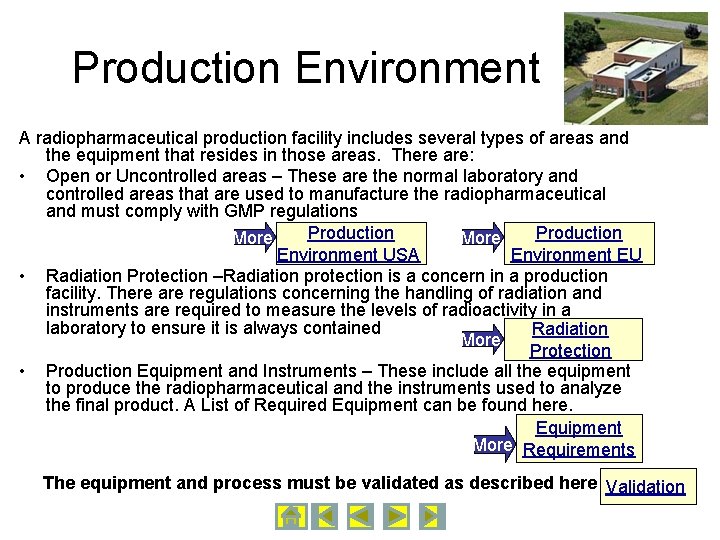
Production Environment A radiopharmaceutical production facility includes several types of areas and the equipment that resides in those areas. There are: • Open or Uncontrolled areas – These are the normal laboratory and controlled areas that are used to manufacture the radiopharmaceutical and must comply with GMP regulations Production More Environment USA Environment EU • Radiation Protection –Radiation protection is a concern in a production facility. There are regulations concerning the handling of radiation and instruments are required to measure the levels of radioactivity in a laboratory to ensure it is always contained Radiation More Protection • Production Equipment and Instruments – These include all the equipment to produce the radiopharmaceutical and the instruments used to analyze the final product. A List of Required Equipment can be found here. Equipment More Requirements The equipment and process must be validated as described here Validation

Validation • • The first step in validation is to create a validation master plan (VMP). The VMP documents the way the facility will operate, who has control over the various aspects of the validation activities, and how production, quality control, and personnel management will be directed. To learn more about the VMP, follow the arrow. Validation More Master Plan All the equipment used in the manufacture of any radiopharmaceutical must be validated to be sure that it will give accurate and meaningful results for all the tests required before the product is released for human use. This includes being sure that the equipment is designed to perform the tests, that it is installed properly, that it is operating properly and that it is performing properly. This is part of the QA function. To learn more about Equipment Validation Specifics, follow the arrow Equipment More Validation To learn about Quality Assurance and Quality Management, see Quality More Management Some tests such as the sterility test cannot be completed before the product is delivered. Because of this, it is very important that the whole process be validated. Read more about process validation. Process More Validation

Cyclotron and Targetry Navigation Tree Cyclotron History Cyclotron Operations Cyclotron Basics Commercial Cyclotrons Cyclotron and Targetry Nuclear Reactions Target Physics Cyclotron Targetry Radionuclide Transfer Beam Heating Density Reduction Target Foils Practical Target Design You may continue to the next slide to learn more about Cyclotrons and Targetry or jump directly to a presentation by clicking on the name.

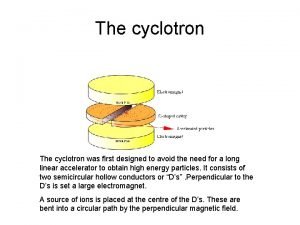
Cyclotron and Targetry • • • The cyclotron is a particle accelerator which is used to produce the radionuclides. It accelerates a charged particle to very high energies. In most cases considered here, this charged particle will be a negatively charged hydrogen ion. Learn about cyclotrons here. Cyclotron More Operations Once the charged particle reaches high enough energies, it is directed out of the cyclotron and hits a target material and makes the radionuclide. An example of this process is the reaction of oxygen-18 enriched water with protons to make fluorine-18. Learn more about cyclotron targetry. Cyclotron More Targetry Once the radionuclide is made, it may be extracted from the target material used in the irradiation. In the case of FDG, the F-18 can be extracted from the O-18 water using a resin column. It must then be transferred out of the cyclotron vault and into the chemistry laboratory. This may be a short distance through a wall or a rather long distance depending on the placement of the cyclotron in the facility. Learn more about radionuclide transfer. Radionuclide More Transfer

FDG Production Navigation Plan You may continue to the next slide to learn more about FDG production or jump directly to a presentation by clicking on the name. FDG Production FDG Synthesizers FDG Synthesis Chemistry How FDG Synthesizers Work Troubleshooting Guide

FDG Production In order to produce a radiopharmaceutical, it necessary to set up a laboratory to accommodate all the equipment and personnel, and to carry out production in a safe and clean environment. The laboratory usually will usually contain: – General laboratory equipment for usual chemical operations as described in the Facility and Equipment section – Analytical equipment to analyze the final product to ensure it is safe and meets all standards of purity and identity as described in the Facility and Equipment section – Production equipment to produce the radiopharmaceutical. This is usually a synthesis module and there are several for FDG by various manufacturers. More information about the synthesis of FDG, the operation of the synthesizers and the synthesizers available can be found by following the arrow More FDG Synthesizers

You may continue to the next slide to learn more about Quality Control Testing and Product Release or jump directly to a presentation by clicking on the name. Quality Control Testing Product Release Navigation Tree This tutorial describes processes and procedures that may be used in the preparation of PET radiopharmaceuticals. Other processes and procedures that are not described in this tutorial may also be acceptable Quality Control Testing Product Release GMP Regulations Quality Control Product Release Shipping IAEA Pub 1225

Quality Control Testing Product Release • • • Quality Control of the final product is one of the most critical steps in the production of the radiopharmaceutical. It is where the final determination is made as to whether or not the radiopharmaceutical can be used in a patient. There are extensive regulations from different governing bodies which govern these tests and regulate production. The regulations can be found here. GMP More Regulations The testing that is done for PET radiopharmaceuticals has to be done quickly since the half-life of the radiopharmaceutical is so short. There a series of very specific tests which must be carried out. These include tests of both chemical and radiochemcial purity, conformation of radiochemical identity, p. H, sterility and to ensure the dose does not contain dangerous levels of pryogens. More information about these tests and a discussion of the methods can be found by following the arrow. Quality More Control Before a product can be released for clinical use, it must approved. This can only be done by an authorized person (qualified person). To learn more about the qualifications necessary and the formal documents that are used to document the release, follow the arrow. Product More Release

Return to the main menu
 Radiopharmaceutical chemistry
Radiopharmaceutical chemistry What are radiopharmaceuticals
What are radiopharmaceuticals Nevada radiation control program
Nevada radiation control program Cyclotron and synchrotron radiation
Cyclotron and synchrotron radiation Pre-production, production, post-production
Pre-production, production, post-production Cyclotron formulas
Cyclotron formulas Cyclotron
Cyclotron Cmgi uc davis
Cmgi uc davis Baby cyclotron
Baby cyclotron Cyclotron
Cyclotron Cyclotron diagram
Cyclotron diagram Cyclotron
Cyclotron Servsafe chapter 10
Servsafe chapter 10 Clean public areas, facilities and equipment
Clean public areas, facilities and equipment Stock receiving and issuing procedures
Stock receiving and issuing procedures Hotel sizes classifications
Hotel sizes classifications Iso 17025 facilities and environmental conditions
Iso 17025 facilities and environmental conditions Design options for a distribution network
Design options for a distribution network Sanitary facilities and equipment
Sanitary facilities and equipment Facility planning
Facility planning Providing support services facilities and other amenities
Providing support services facilities and other amenities Translators and facilities of languages
Translators and facilities of languages Safe facilities and pest management
Safe facilities and pest management