Quiz 3 Quiz 2 answers 1 What area












- Slides: 26

Quiz 3 •

Quiz 2 answers 1. What area of mathematics made the kinetic theory of gases possible? Statistical Mechanics or Statistics 2. Describe the significance of Brownian motion. It shows that there atoms that exist. That are invisible to the eye or under a microscope but can be shown to affect larger molecules (i. e. pollen grains). 3. What two laws combined to develop the ideal gas law? Charles and Boyles Law 4. Why are units important To check your work for accuracy or reasonableness

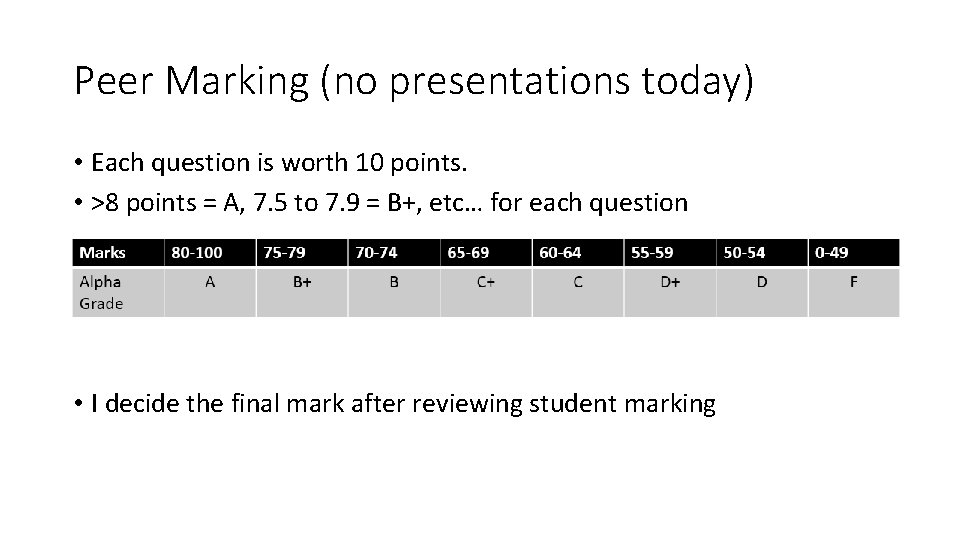
Peer Marking (no presentations today) • Each question is worth 10 points. • >8 points = A, 7. 5 to 7. 9 = B+, etc… for each question • I decide the final mark after reviewing student marking

Marking Guidance • Qualitative questions • Is the physical concept portrayed accurately? • Is it clearly explained or answered? • Quantitative questions • Is there a diagram? • Is the correct equation used? • Are the units handled properly? • Is the equation solved for the unknown algebraically? • Is the solution correct? Are the units included?

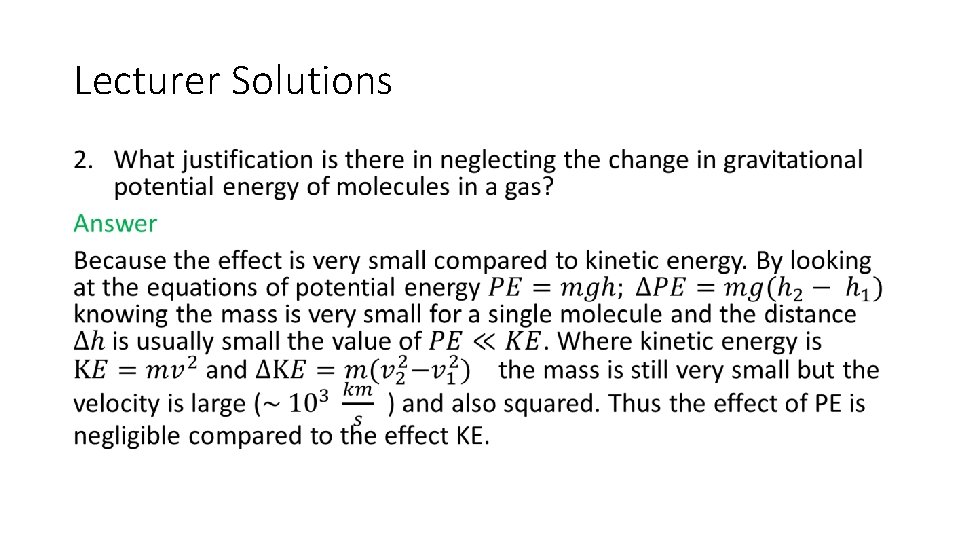
Lecturer Solutions 1. In discussing the fact that it is impossible to apply the laws of mechanics individually to atoms in a macroscopic system, Mayer states: “The very complexity of the problem [that is, the fact that the number of atoms is large] is the secret of its solution. ” discuss this sentence. Answer Because in gases the number of atoms is very large, statistical methods (e. g. Maxwell distributions, averages, means, etc. ) can be applied to the describe the behavior of a ‘typical’ atom or atoms

Lecturer Solutions •

Lecturer Solutions •

Lecturer Solutions •

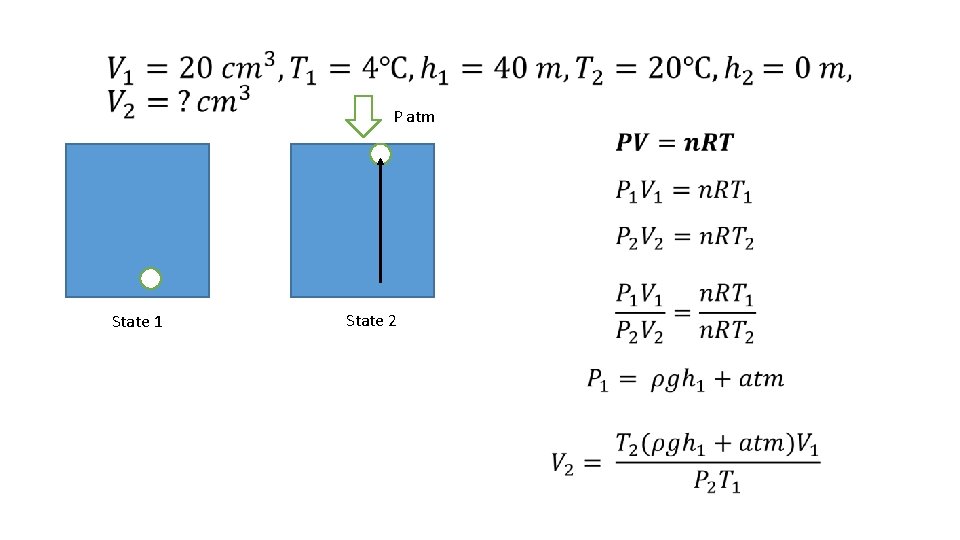
P atm State 1 State 2 •


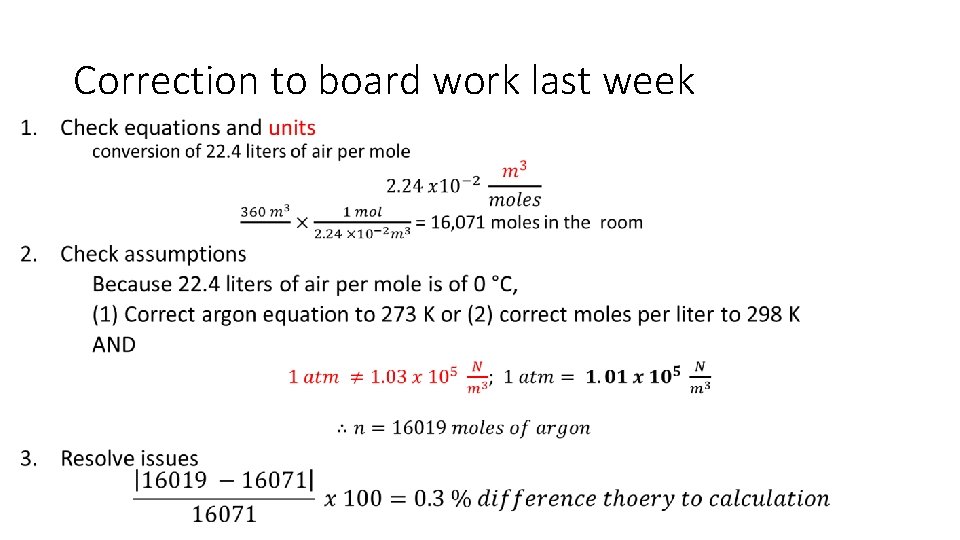
Correction to board work last week •

Everyone gets full credit (10 points) for attempting corrections to board work

Finish marking you papers and hand them forward for my review

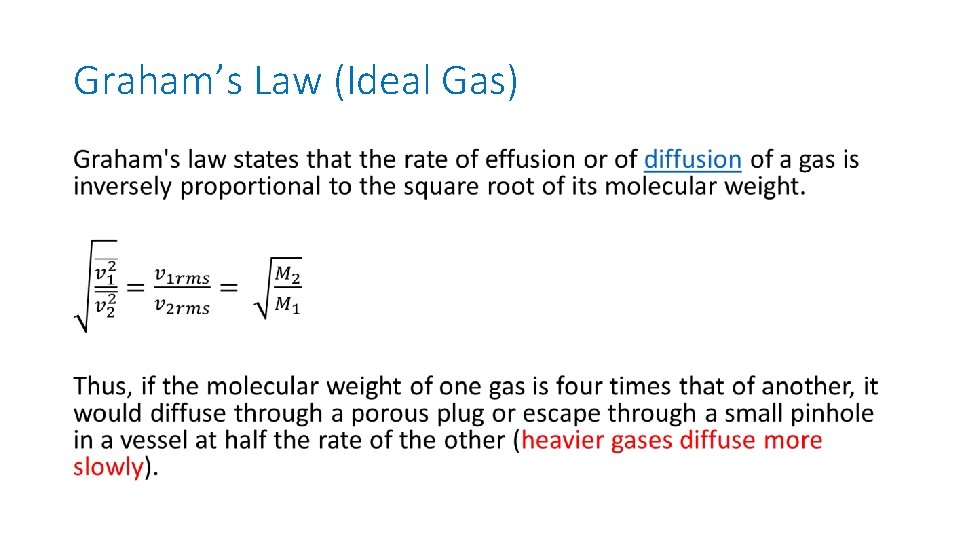
Graham’s Law (Ideal Gas) •

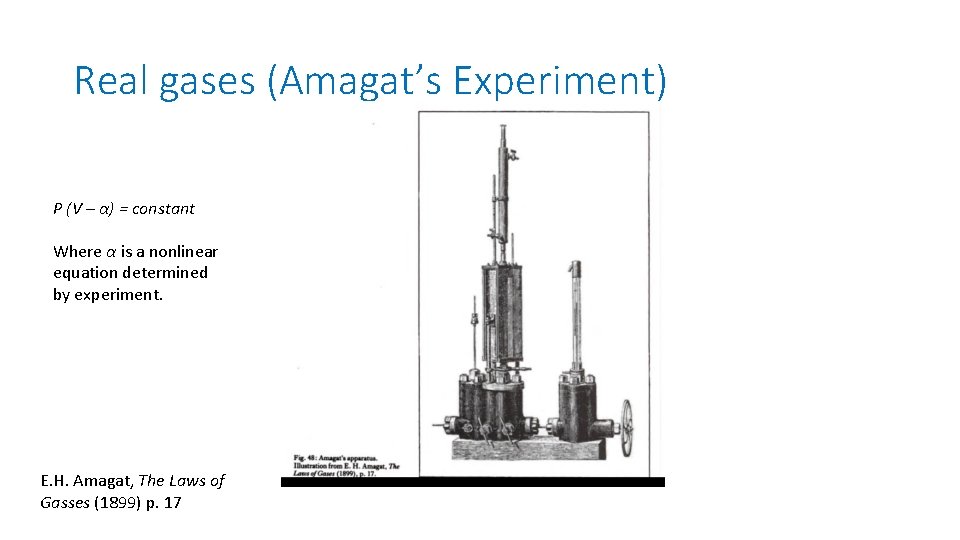
Real gases (Amagat’s Experiment) P (V – α) = constant Where α is a nonlinear equation determined by experiment. E. H. Amagat, The Laws of Gasses (1899) p. 17

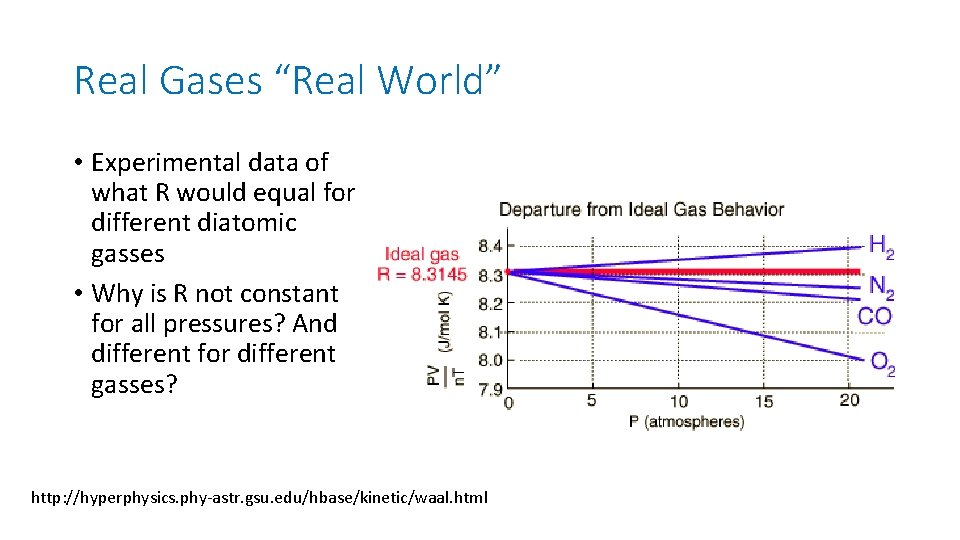
Real Gases “Real World” • Experimental data of what R would equal for different diatomic gasses • Why is R not constant for all pressures? And different for different gasses? http: //hyperphysics. phy-astr. gsu. edu/hbase/kinetic/waal. html

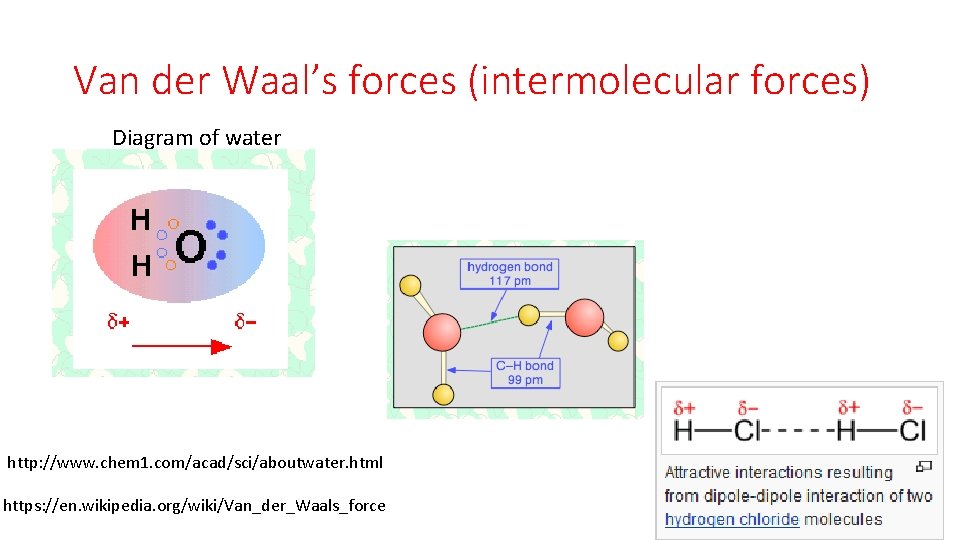
Van der Waal’s forces (intermolecular forces) Diagram of water http: //www. chem 1. com/acad/sci/aboutwater. html https: //en. wikipedia. org/wiki/Van_der_Waals_force

Van der Waal’s forces • Van der Waals forces include attractions and repulsions between atoms, molecules, and surfaces, as well as other intermolecular forces. They differ from covalent and ionic bonding in that they are caused by correlations in the fluctuating polarizations of nearby particles (a consequence of quantum dynamics[4]). https: //en. wikipedia. org/wiki/Van_der_Waals_force

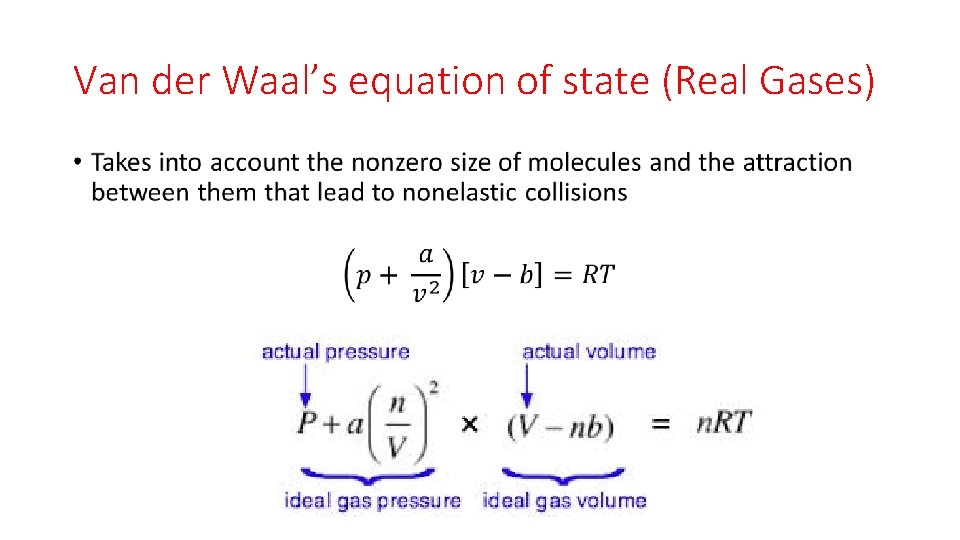
Van der Waal’s equation of state (Real Gases) •

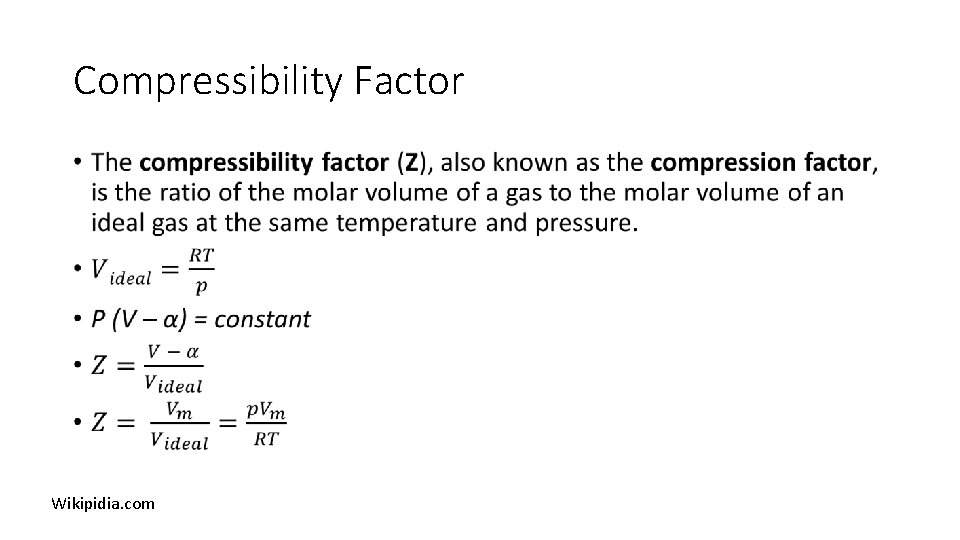
Compressibility Factor • Wikipidia. com


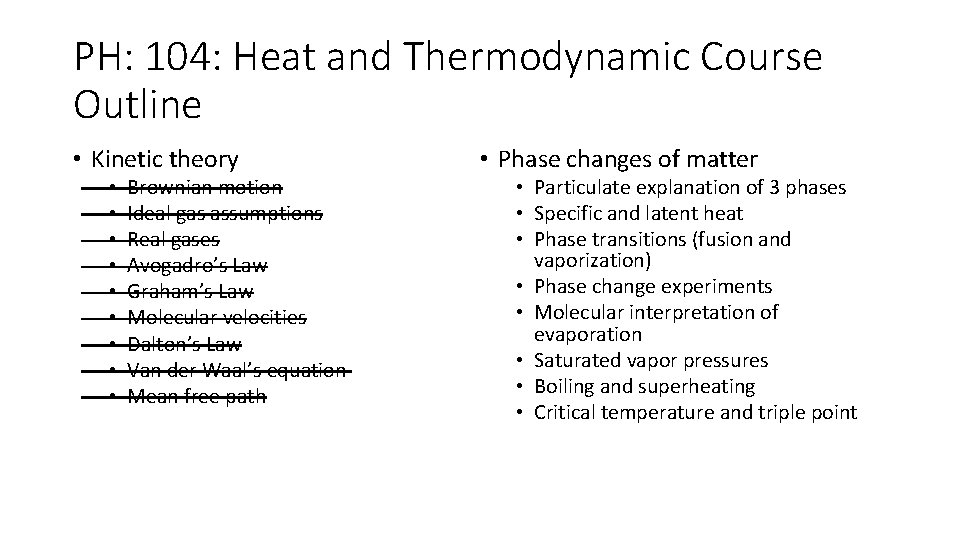
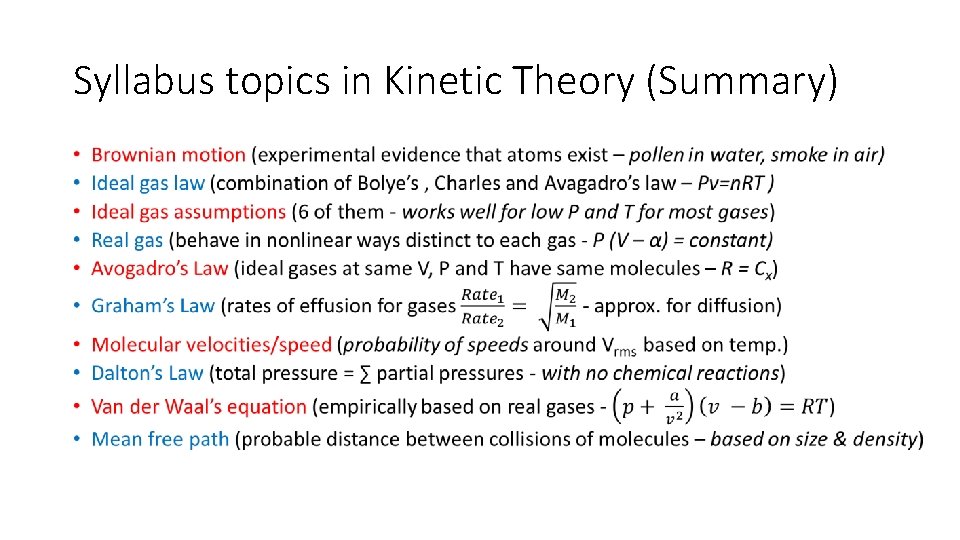
PH: 104: Heat and Thermodynamic Course Outline • Kinetic theory • • • Brownian motion Ideal gas assumptions Real gases Avogadro’s Law Graham’s Law Molecular velocities Dalton’s Law Van der Waal’s equation Mean free path • Phase changes of matter • Particulate explanation of 3 phases • Specific and latent heat • Phase transitions (fusion and vaporization) • Phase change experiments • Molecular interpretation of evaporation • Saturated vapor pressures • Boiling and superheating • Critical temperature and triple point

Syllabus topics in Kinetic Theory (Summary) •

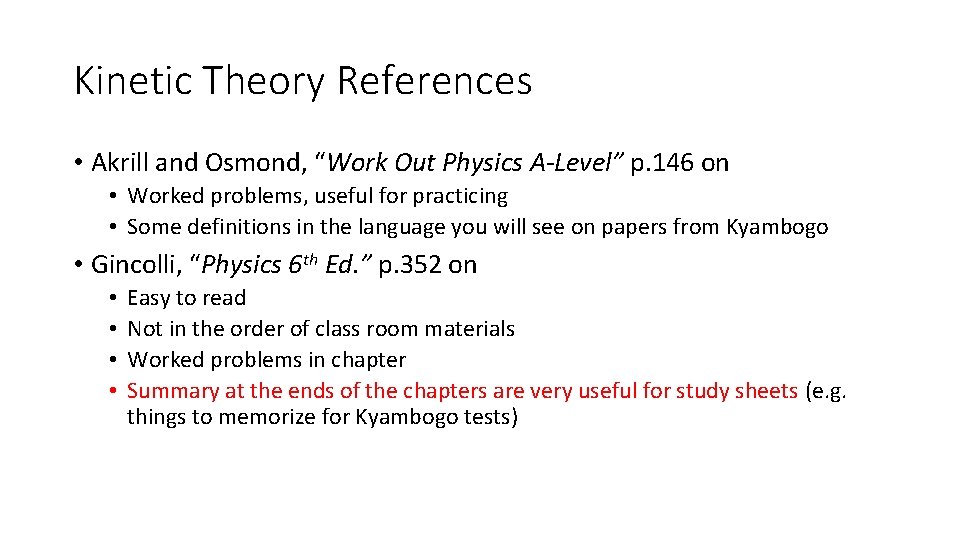
Kinetic Theory References • Akrill and Osmond, “Work Out Physics A-Level” p. 146 on • Worked problems, useful for practicing • Some definitions in the language you will see on papers from Kyambogo • Gincolli, “Physics 6 th Ed. ” p. 352 on • • Easy to read Not in the order of class room materials Worked problems in chapter Summary at the ends of the chapters are very useful for study sheets (e. g. things to memorize for Kyambogo tests)

Review topics • Particulate explanation of 3 phases (+2) • Specific and latent heat • Phase transitions (fusion, vaporization, etc. ) • Phase change experiments (calorimeters, etc. )

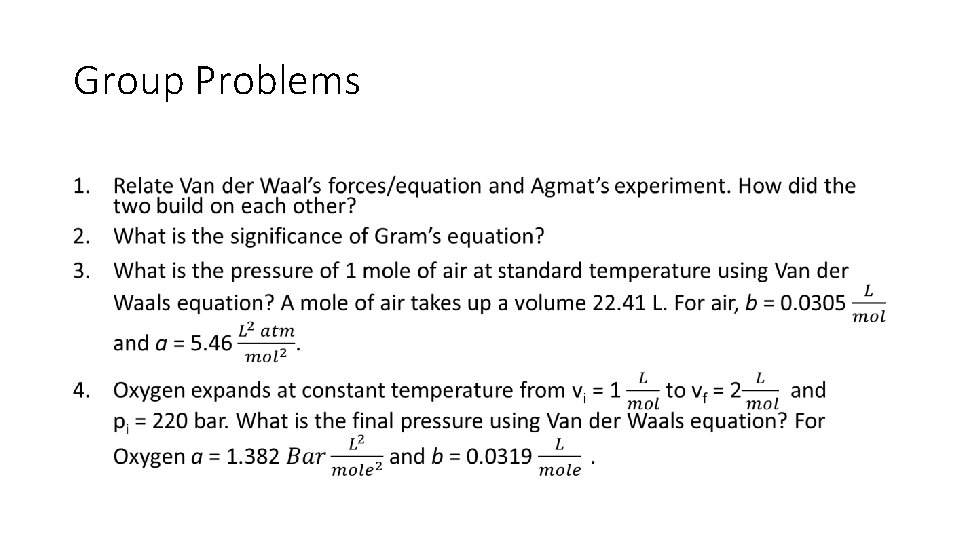
Group Problems •
 Formula for sector area
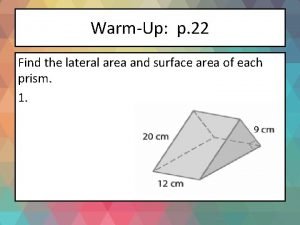
Formula for sector area What is the lateral surface area
What is the lateral surface area What is structured programming language
What is structured programming language Ibm system/390
Ibm system/390 Surface area of a cone
Surface area of a cone Lateral area and surface area
Lateral area and surface area Cobol cheat sheet
Cobol cheat sheet Gastrulation in fish
Gastrulation in fish Area nervina area radicularis
Area nervina area radicularis Surface area vs area
Surface area vs area Denture landmarks
Denture landmarks Lateral edge
Lateral edge Lateral area
Lateral area Area radicularis
Area radicularis Systema nervosum periphericum
Systema nervosum periphericum Names of prisms
Names of prisms How to find the surface area of an octagonal prism
How to find the surface area of an octagonal prism 10-4 surface area of prisms and cylinders answers
10-4 surface area of prisms and cylinders answers Surface area of a pyramid
Surface area of a pyramid What is this?
What is this? 11-2 volumes of prisms and cylinders
11-2 volumes of prisms and cylinders Circle-area worksheet answers
Circle-area worksheet answers Area of regular polygons maze answers
Area of regular polygons maze answers 10-4 surface area of prisms and cylinders
10-4 surface area of prisms and cylinders Mathswatch answers 2021
Mathswatch answers 2021 Similar shapes area worksheet
Similar shapes area worksheet Definition of composite figure in math
Definition of composite figure in math