PROPERTIES OF WATER WATER COVERS OF EARTHS SURFACE












- Slides: 18

PROPERTIES OF WATER

WATER • COVERS ¾ OF EARTH’S SURFACE • MOST ABUNDANT COMPOUND IN LIVING THINGS • LIQUID AT THE TEMPS FOUND OVER MUCH OF THE EARTH’S SURFACE • EXPANDS AS IT FREEZES • ICE FLOATS


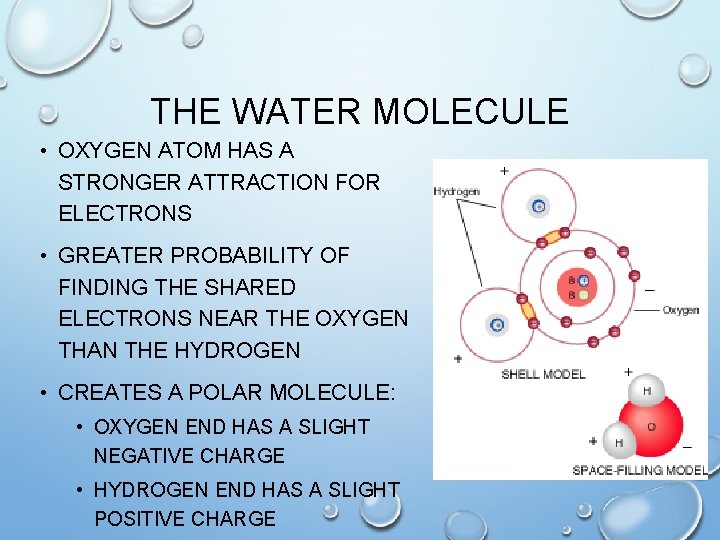
THE WATER MOLECULE • OXYGEN ATOM HAS A STRONGER ATTRACTION FOR ELECTRONS • GREATER PROBABILITY OF FINDING THE SHARED ELECTRONS NEAR THE OXYGEN THAN THE HYDROGEN • CREATES A POLAR MOLECULE: • OXYGEN END HAS A SLIGHT NEGATIVE CHARGE • HYDROGEN END HAS A SLIGHT POSITIVE CHARGE

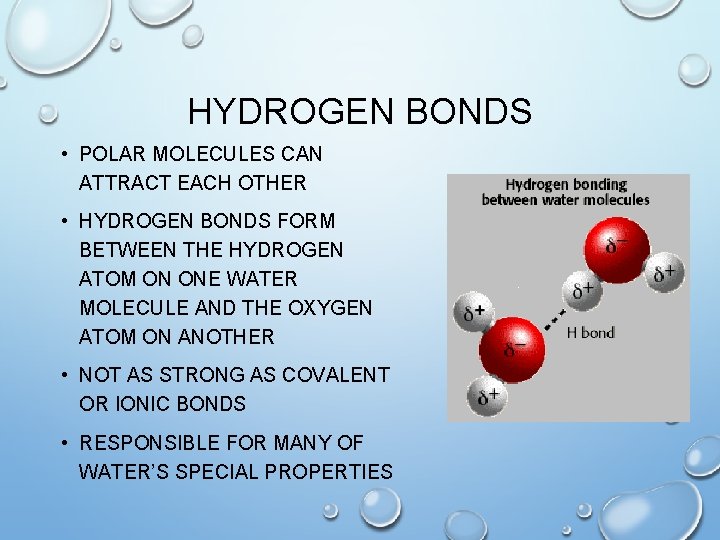
HYDROGEN BONDS • POLAR MOLECULES CAN ATTRACT EACH OTHER • HYDROGEN BONDS FORM BETWEEN THE HYDROGEN ATOM ON ONE WATER MOLECULE AND THE OXYGEN ATOM ON ANOTHER • NOT AS STRONG AS COVALENT OR IONIC BONDS • RESPONSIBLE FOR MANY OF WATER’S SPECIAL PROPERTIES


COHESION • ATTRACTION BETWEEN MOLECULES OF THE SAME SUBSTANCE • WATER’S COHESION CAUSES MOLECULES ON THE SURFACE OF WATER TO BE DRAWN INWARD • FORMING BEADS • THIS “SURFACE TENSION” ALLOWS SOME INSECTS AND SPIDERS TO WALK ON A POND’S SURFACE

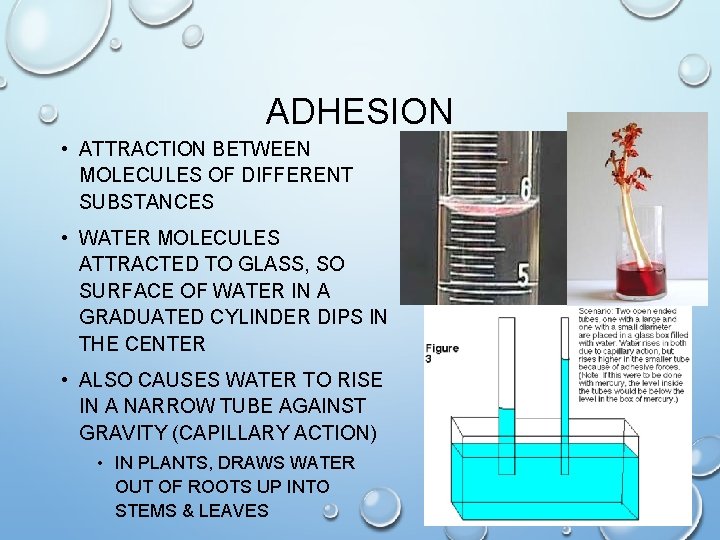
ADHESION • ATTRACTION BETWEEN MOLECULES OF DIFFERENT SUBSTANCES • WATER MOLECULES ATTRACTED TO GLASS, SO SURFACE OF WATER IN A GRADUATED CYLINDER DIPS IN THE CENTER • ALSO CAUSES WATER TO RISE IN A NARROW TUBE AGAINST GRAVITY (CAPILLARY ACTION) • IN PLANTS, DRAWS WATER OUT OF ROOTS UP INTO STEMS & LEAVES


MIXTURE • COMPOSED OF TWO OR MORE ELEMENTS OR COMPOUNDS THAT ARE PHYSICALLY MIXED TOGETHER BUT NOT CHEMICALLY COMBINED • SALT & PEPPER • SUGAR & SAND • EARTH’S ATMOSPHERE • TWO TYPES OF MIXTURES WITH WATER solutions suspensions

SOLUTIONS • MIXTURE OF 2 OR MORE SUBSTANCES IN WHICH THE MOLECULES ARE EVENLY DISTRIBUTED • SOLUTE: SUBSTANCE THAT IS DISSOLVED • SOLVENT: SUBSTANCE IN WHICH THE SOLUTE DISSOLVED • WATER: GREATEST SOLVENT ON EARTH • DUE TO ITS POLARITY

SUSPENSIONS • MIXTURES OF WATER AND NONDISSOLVED MATERIAL • BLOOD: A SOLUTION AND A SUSPENSION • MOSTLY WATER WITH MANY DISSOLVED COMPOUNDS • CONTAINS CELLS & OTHER UNDISSOLVED PARTICLES THAT REMAIN IN SUSPENSION


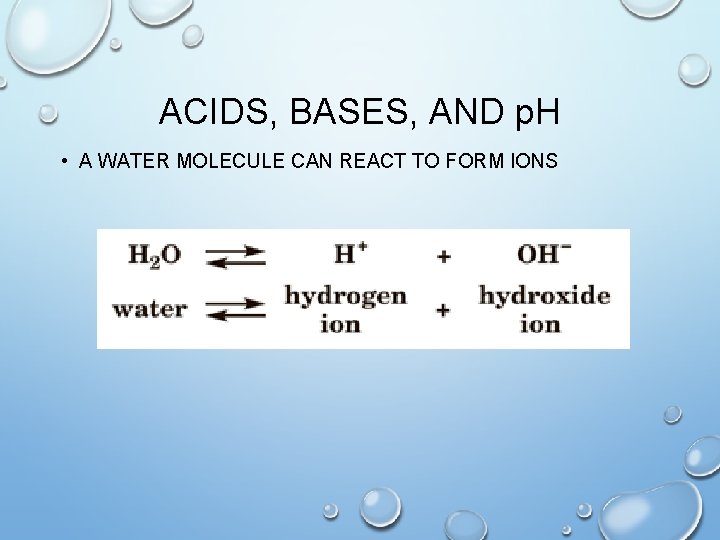
ACIDS, BASES, AND p. H • A WATER MOLECULE CAN REACT TO FORM IONS

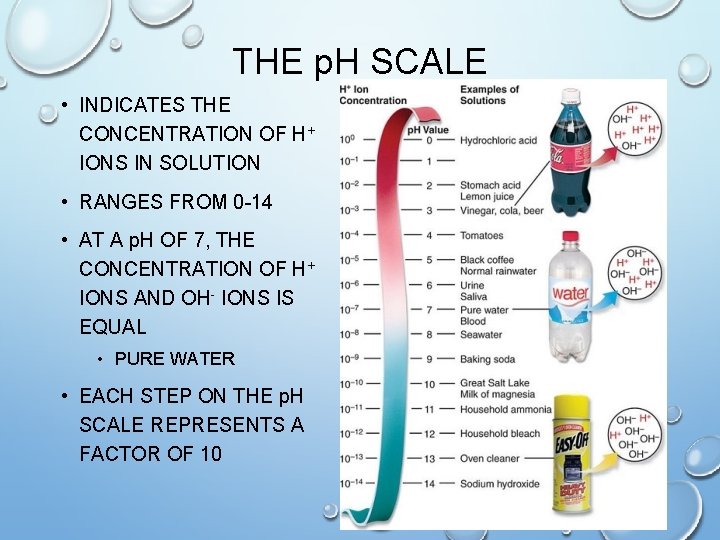
THE p. H SCALE • INDICATES THE CONCENTRATION OF H+ IONS IN SOLUTION • RANGES FROM 0 -14 • AT A p. H OF 7, THE CONCENTRATION OF H+ IONS AND OH- IONS IS EQUAL • PURE WATER • EACH STEP ON THE p. H SCALE REPRESENTS A FACTOR OF 10

ACIDS • ANY COMPOUND THAT FORMS H+ IONS IN SOLUTION • HIGHER CONCENTRATION OF H+ IONS THAN PURE WATER • p. H VALUES BELOW 7 • THE FARTHER AWAY FROM 7, THE STRONGER THE ACID IS

BASES • COMPOUND THAT PRODUCES HYDROXIDE (OH-) IONS IN SOLUTION • ALSO CALLED ALKALINE • CONTAIN LOWER CONCENTRATIONS OF H+ IONS THAN PURE WATER • p. H VALUES ABOVE 7 • THE FARTHER AWAY FROM 7, THE STRONGER THE BASE IS

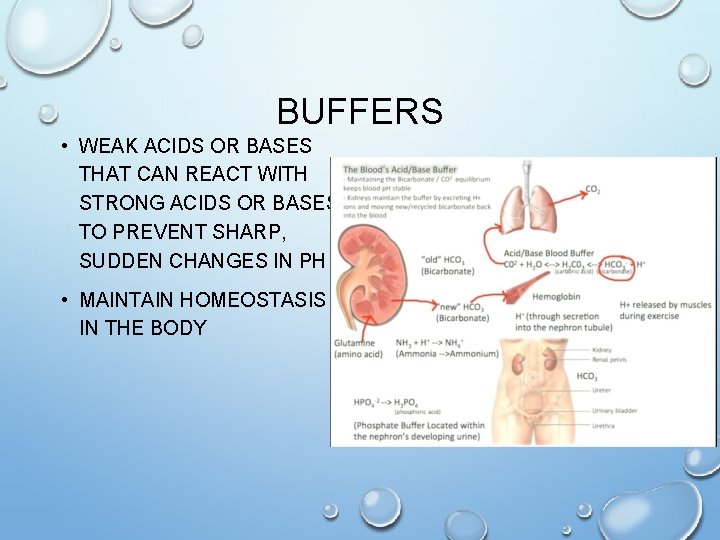
BUFFERS • WEAK ACIDS OR BASES THAT CAN REACT WITH STRONG ACIDS OR BASES TO PREVENT SHARP, SUDDEN CHANGES IN PH • MAINTAIN HOMEOSTASIS IN THE BODY
 Images of hydrosphere
Images of hydrosphere Kerri donaldson
Kerri donaldson Water and water and water water
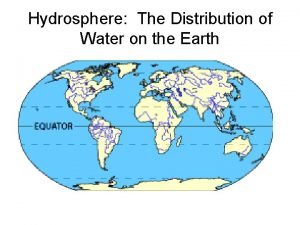
Water and water and water water 71 of earth is covered with water
71 of earth is covered with water An ecosystem in which water either covers the soil
An ecosystem in which water either covers the soil Water covers approximately
Water covers approximately Water covers about
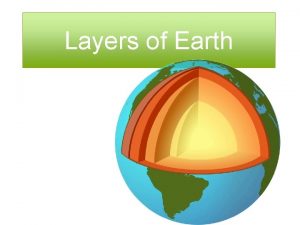
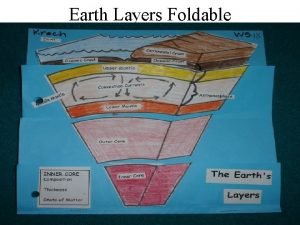
Water covers about The earths layer foldable
The earths layer foldable Earths roation
Earths roation Whats a natural satellite
Whats a natural satellite Biome near the equator
Biome near the equator What are the families of the periodic table
What are the families of the periodic table How thick is the earths crust
How thick is the earths crust Whats earths moon called
Whats earths moon called Which layer of the earth slowly moves like putty
Which layer of the earth slowly moves like putty Earths early atmosphere contained
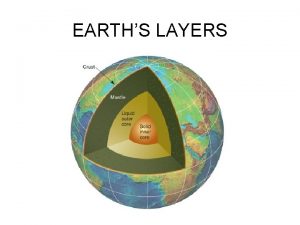
Earths early atmosphere contained Middle layer of earth
Middle layer of earth Earths major crustal plates
Earths major crustal plates Earths orbit seasons
Earths orbit seasons