Properties of Water Water covers 23 of Earth


























- Slides: 29

Properties of Water

Water covers 2/3 of Earth • 97% Saltwater oceans • 2. 4% Ice (polar caps and glaciers) • 0. 6% Surface water (lakes and streams)


The Human body is made of ~80% water. • To function properly, the body requires between one and seven liters of water per day to avoid dehydration; the precise amount depends on the level of activity, temperature, humidity, and other factors. • Most of this is ingested through foods or beverages other than drinking straight water.

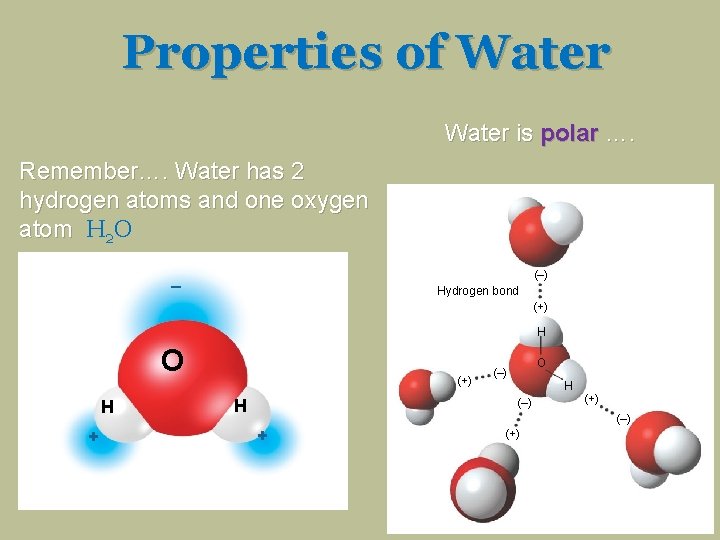
Properties of Water is polar …. Remember…. Water has 2 hydrogen atoms and one oxygen atom H 2 O _ (–) Hydrogen bond (+) H O H + (+) H O (–) H (–) (+) (–) + (+)

Properties of Water cohesion adhesion High specific heat density p. H

Cohesion • The natural attraction of a water molecule to other water molecule is called cohesion.


Adhesion • The attraction of a water molecule to another polar molecule is adhesion. water droplets on a spider’s web (polar surface).

Properties associated with polarity/ adhesion, cohesion • Capillary Action • Surface Tension • Transpiration

Capillary Action

Surface Tension • At the surface of the drop of water, water does not attract to the air…. . Surface tension

Jesus Lizard

Transpiration

Properties of Water cohesion adhesion density High specific heat p. H

Density


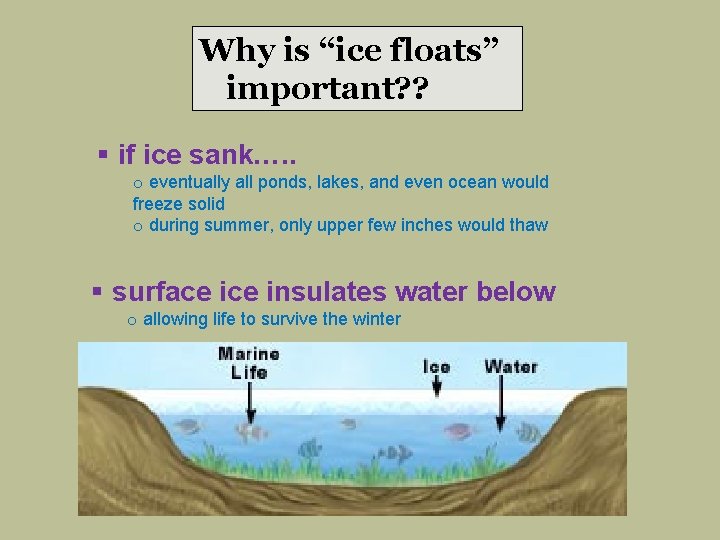
Why is “ice floats” important? ? § if ice sank…. . o eventually all ponds, lakes, and even ocean would freeze solid o during summer, only upper few inches would thaw § surface insulates water below o allowing life to survive the winter

Properties of Water cohesion adhesion density High specific heat p. H



An example is the more moderate climate of a coastal location compared to one far inland.

Properties of Water cohesion adhesion density High specific heat p. H


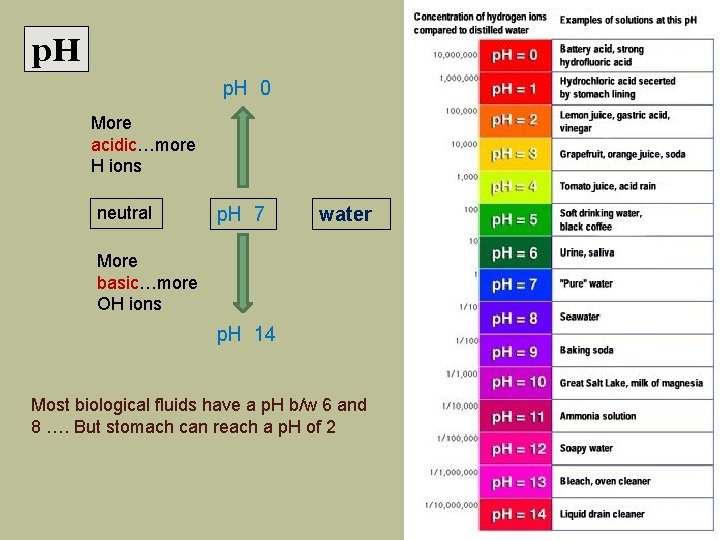
p. H 0 More acidic…more H ions neutral p. H 7 water More basic…more OH ions p. H 14 Most biological fluids have a p. H b/w 6 and 8 …. But stomach can reach a p. H of 2

Properties of Water cohesion adhesion density High specific heat p. H



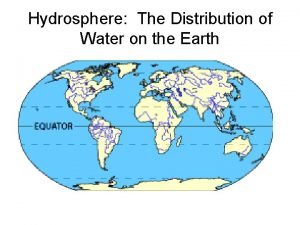
 How much water covers the earth
How much water covers the earth Hydrosphere distribution
Hydrosphere distribution Water and water and water water
Water and water and water water An ecosystem in which water either covers the soil
An ecosystem in which water either covers the soil Water covers approximately
Water covers approximately Water covers about
Water covers about Properties of outer core
Properties of outer core Families of elements
Families of elements Intensive property and extensive properties
Intensive property and extensive properties Physical property and chemical property
Physical property and chemical property Numeracy stages
Numeracy stages The curved part of head with mechanism for needle operation
The curved part of head with mechanism for needle operation Membrane covering the heart
Membrane covering the heart Magazine back cover design
Magazine back cover design Companion diagnostic
Companion diagnostic Back cover of hatchet
Back cover of hatchet Methods for salvage covers
Methods for salvage covers Thriller dvd covers
Thriller dvd covers Chapter 15 digestive system
Chapter 15 digestive system Which layer of the osi model includes vlans
Which layer of the osi model includes vlans Purpose of magazine covers
Purpose of magazine covers Components of the reflex arc
Components of the reflex arc Whats tone in literature
Whats tone in literature Blood covers sin
Blood covers sin Robinson crusoe book covers
Robinson crusoe book covers What covers mercury's surface
What covers mercury's surface What is the purpose of a magazine cover
What is the purpose of a magazine cover What is the purpose of magazine cover
What is the purpose of magazine cover Lines body cavities
Lines body cavities Achievements of rntcp
Achievements of rntcp