Properties of Light Wave properties and the interaction
















- Slides: 20

Properties of Light Wave properties and the interaction of light with other materials

What is Light? • Light’s composition has been debated for centuries. Light exhibits properties of waves, but also behaves like a stream of particles. During 17 th century, rectilinear propagation, reflection and refraction were three most commonly observed wave properties. • Two different theories emerged to explain these properties. The first, corpuscular theory, was put forth by Isaac Newton.

• Corpuscular theory viewed light as a stream of particles, and explained the observable wave properties as follows: • Rectilinear propagation- Light travels in straight lines. A particle, like a ball, can be thrown in a straight line, defying gravity. (Over short distances). This idea made a strong argument for particle theory. • An argument against a wave theory involved comparison to sound. Sound can be heard around an obstacle while a stream of light cannot be seen from behind an obstacle.

• Reflection could be simulated easily enough by bouncing ball bearings off of a steel plate (Θi = Θr). If corpuscles are elastic, light would behave same way, which it did when reflecting.

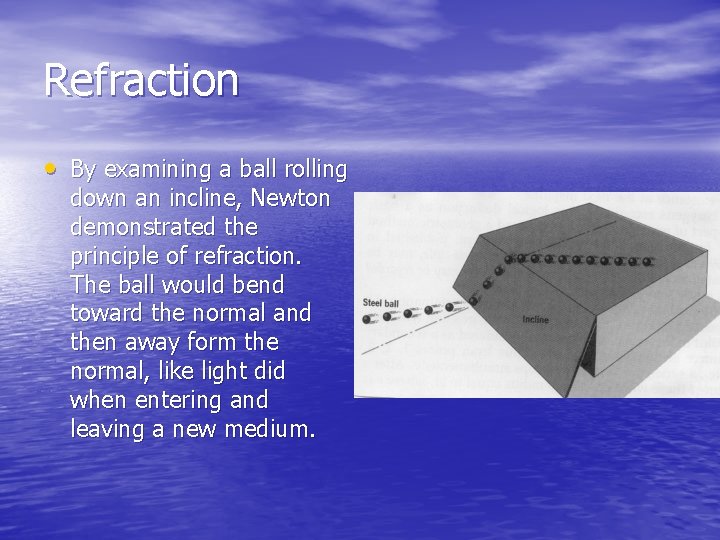
Refraction • By examining a ball rolling down an incline, Newton demonstrated the principle of refraction. The ball would bend toward the normal and then away form the normal, like light did when entering and leaving a new medium.

• Newton’s plausible explanations and demonstrations, along with his prestige, gave corpuscular theory the upper hand for many years. His theory dominates for almost 150 years after his death until about 1850.

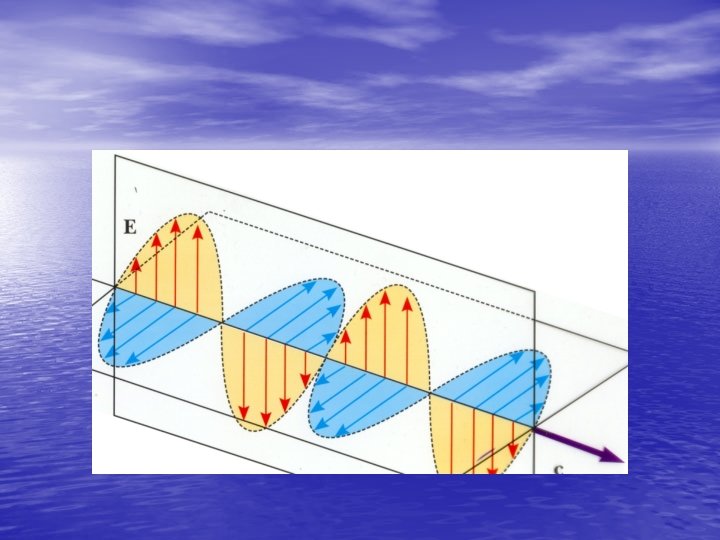
Wave Theory • Wave theory, put forth primarily by Huygens, treats light as a train of waves having wave fronts perpendicular to the direction of travel, and represented by rays. Huygens thought each point on a wave front propagated the movement of the next point on the wave front.

• Reflection and refraction were easily explained by wave theorists, and before 1800, interference of light was not known to exist. The biggest factor in shaping the accepted theory of light came about 150 years after Newton’s death. • Wave theorist’s explanation of refraction required that light slow down when it entered a more dense medium, like water or glass. Newton’s refraction demo required light to speed up upon entering a new media. Circa 1720, measuring light speed was not possible.

• In 1801, when interference of light was discovered, wave theory had the most probable explanation, and then in 1850, Foucault measured the speed of light in water and found it to be slower than in air, which sounded the death knell for corpuscular theory. • Faraday related heat and electricity to each other and in 1865, Maxwell was able to tie magnetism to light and heat, and then determined that heat and light were both electromagnetic disturbances that traveled as waves.

• Finally in 1885, Hertz was able to show that transmitted light and generated electricity are of the same wave nature.


• This allowed the explanation of all observable optical properties of light. This electromagnetic energy has different properties based upon frequency differences.

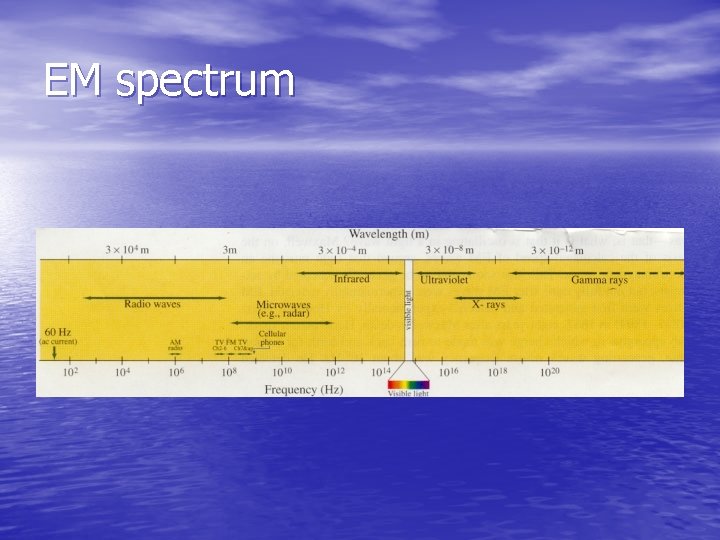
EM spectrum

• There are eight regions of the EM spectrum. • Hard Gamma Rays- Extremely High energy protons in space. When they collide with atmospheric nuclei, showers of high energy particles descend. • Gamma rays- high energy photons of enormous penetrating power from radioactive decay of nuclei. • X-Rays- high energy radiations produced by streams of electrons bombarding a metal plate in a vacuum.

• Ultra-violet radiation- from the sun. Absorbed in our atmosphere, has germicidal, photochemical and fluorescent effects. • Visible spectrum- the light we see • Infrared radiation- used for heating and drying • Radio waves- cover broad band of spectrum used for communications • Power frequencies- produced by electric generators and transmitted by power lines.

• Several scientists found that illuminating metal plates with certain frequencies of light would cause the plates to acquire a positive charge, or lose electrons. When the light was increased in intensity, the rate of loss of electrons would increase. It was also found that for a particular plate, there existed a minimum frequency of light required to begin emission of photoelectrons. Light of less than this threshold frequency, but of great intensity would not cause emission.

• Planck used this observation to hypothesize that light contained energy in small packets, or photons, which he called quanta. The size of the quanta was dependent upon the frequency of the light, not the intensity or brightness. • Planck was able to establish that the energy of a photon was proportional to the frequency of light multiplied by a constant. • E = hv where h is Planck’s constant 6. 63 x 10 -34 j·s and v is frequency in Hz

• This idea of quantized energy explained why light below the threshold frequency would not cause emission of photoelectrons, the packets had to be a certain size, and could only be absorbed as a whole packet, not added together or combined.

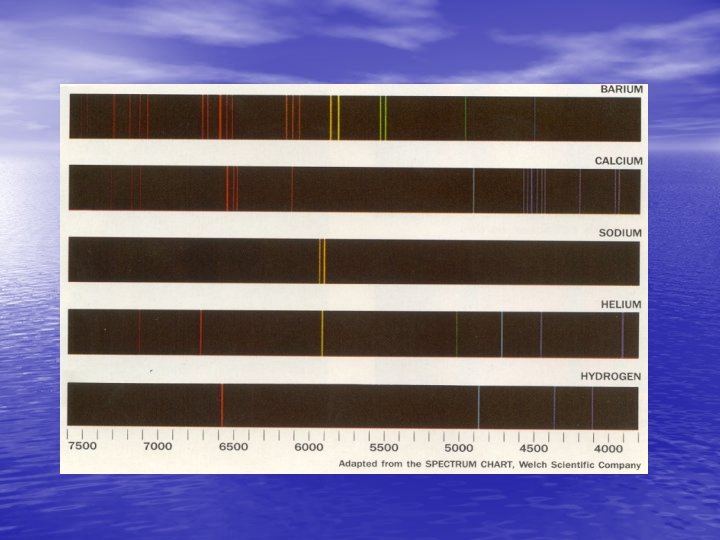
• The modern view of light is : radiant energy that is transported in photons that are guided along their way by electromagnetic waves. Around 1920, it was discovered that electrons in atoms absorb and release energy in quanta resulting in both emission and absorption spectra which are characteristic of each element.

 Light light light chapter 23
Light light light chapter 23 Light light light chapter 22
Light light light chapter 22 Light light light chapter 22
Light light light chapter 22 Forrester real time interaction management
Forrester real time interaction management Difference between full wave and half wave rectifier
Difference between full wave and half wave rectifier Difference between transverse wave and longitudinal wave
Difference between transverse wave and longitudinal wave Full wave rectifier examples
Full wave rectifier examples Determining the arrival times between p-wave and s-wave
Determining the arrival times between p-wave and s-wave What do all waves transmit
What do all waves transmit Mechanical waves characteristics
Mechanical waves characteristics Transverse wave and longitudinal wave example
Transverse wave and longitudinal wave example Light waves are transverse waves true or false
Light waves are transverse waves true or false Long waves and short waves
Long waves and short waves Examples of half wave rectifier
Examples of half wave rectifier Full wave rectified sine wave fourier series
Full wave rectified sine wave fourier series What is a wave
What is a wave Wave wave repeating
Wave wave repeating Fourier series non symmetric interval
Fourier series non symmetric interval Put out that light
Put out that light Membrane-bound organelles
Membrane-bound organelles The bouncing off of light
The bouncing off of light