Product Use Management Grade 1 and Grade 2
- Slides: 12

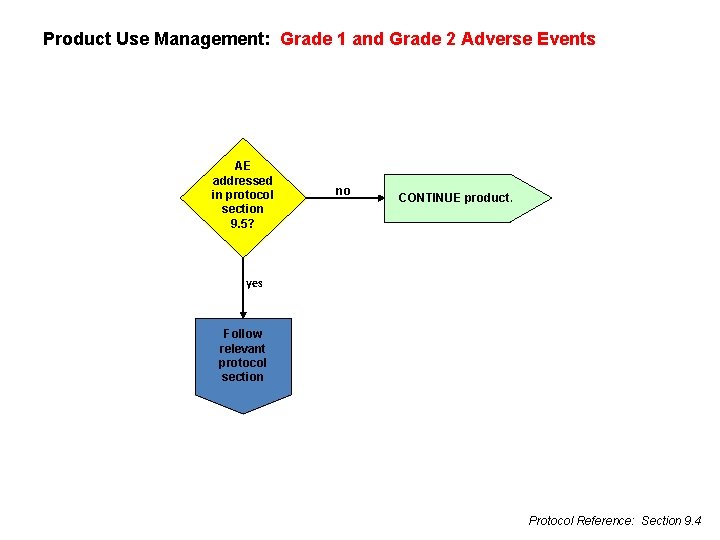
Product Use Management: Grade 1 and Grade 2 Adverse Events AE addressed in protocol section 9. 5? no CONTINUE product. yes Follow relevant protocol section Protocol Reference: Section 9. 4

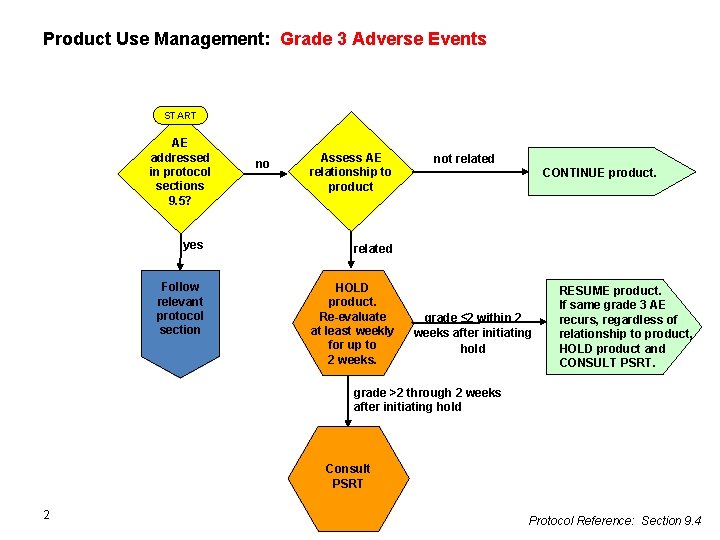
Product Use Management: Grade 3 Adverse Events START AE addressed in protocol sections 9. 5? no Assess AE relationship to product yes related Follow relevant protocol section HOLD product. Re-evaluate at least weekly for up to 2 weeks. not related CONTINUE product. grade ≤ 2 within 2 weeks after initiating hold RESUME product. If same grade 3 AE recurs, regardless of relationship to product, HOLD product and CONSULT PSRT. grade >2 through 2 weeks after initiating hold Consult PSRT 2 Protocol Reference: Section 9. 4

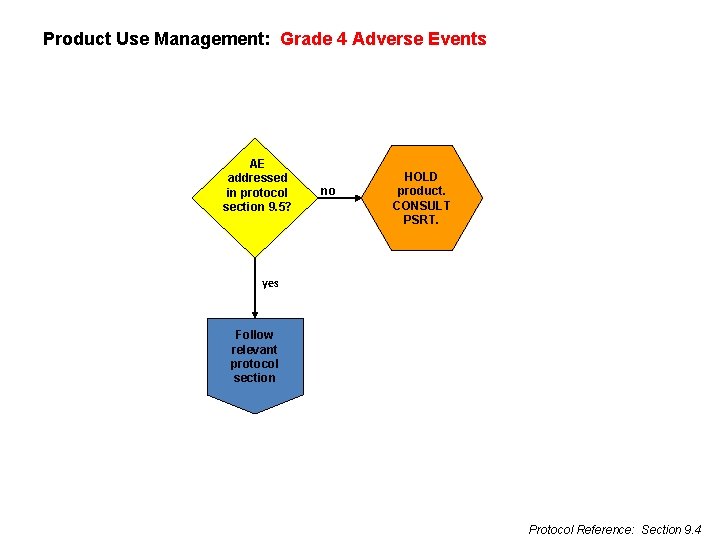
Product Use Management: Grade 4 Adverse Events AE addressed in protocol section 9. 5? no HOLD product. CONSULT PSRT. yes Follow relevant protocol section Protocol Reference: Section 9. 4

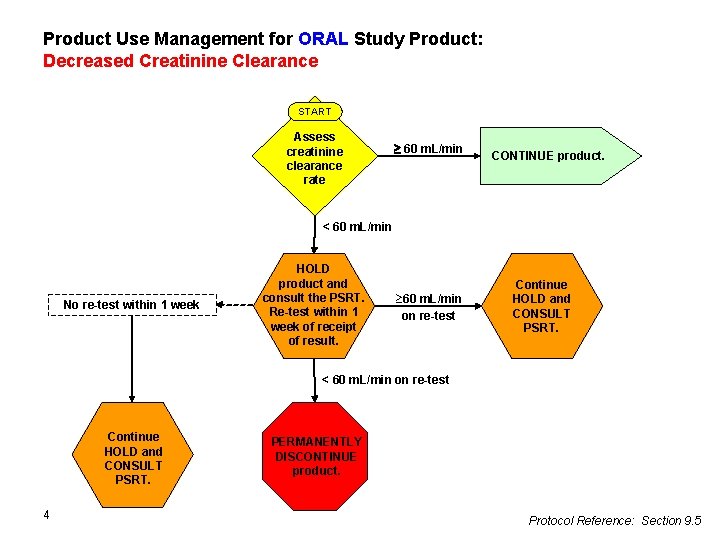
Product Use Management for ORAL Study Product: Decreased Creatinine Clearance START Assess creatinine clearance rate 60 m. L/min CONTINUE product. < 60 m. L/min No re-test within 1 week HOLD product and consult the PSRT. Re-test within 1 week of receipt of result. ³ 60 m. L/min on re-test Continue HOLD and CONSULT PSRT. < 60 m. L/min on re-test Continue HOLD and CONSULT PSRT. 4 PERMANENTLY DISCONTINUE product. Protocol Reference: Section 9. 5

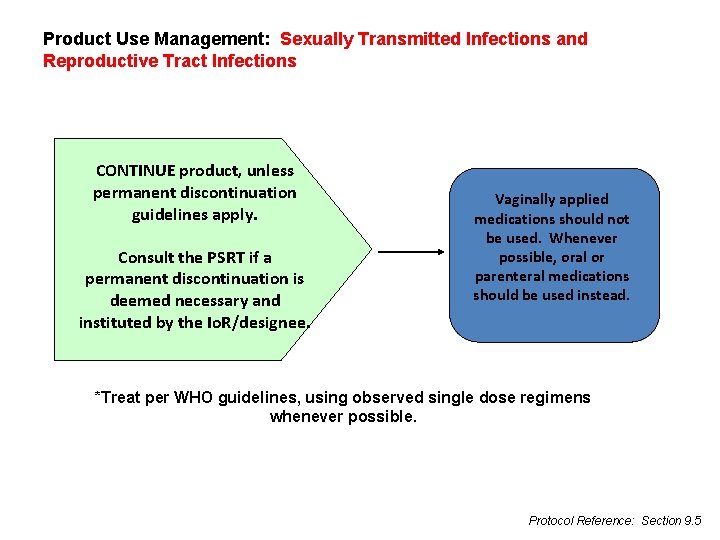
Product Use Management: Sexually Transmitted Infections and Reproductive Tract Infections CONTINUE product, unless permanent discontinuation guidelines apply. Consult the PSRT if a permanent discontinuation is deemed necessary and instituted by the Io. R/designee. Vaginally applied medications should not be used. Whenever possible, oral or parenteral medications should be used instead. *Treat per WHO guidelines, using observed single dose regimens whenever possible. Protocol Reference: Section 9. 5

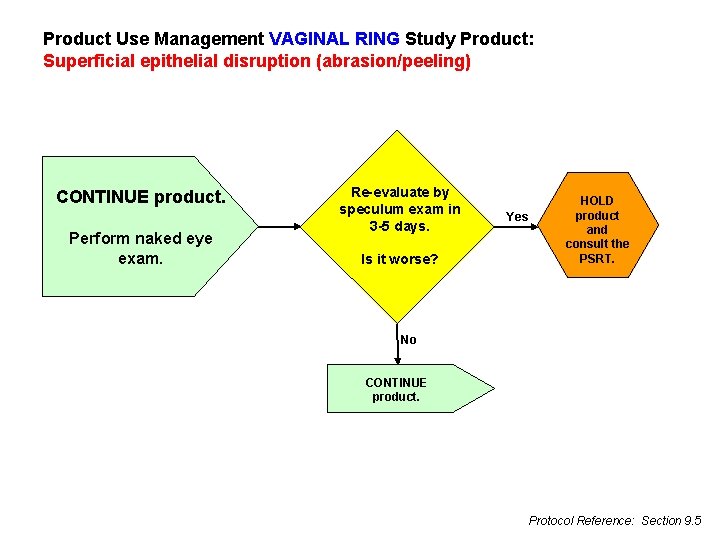
Product Use Management VAGINAL RING Study Product: Superficial epithelial disruption (abrasion/peeling) CONTINUE product. Perform naked eye exam. Re-evaluate by speculum exam in 3 -5 days. Is it worse? Yes HOLD product and consult the PSRT. No CONTINUE product. Protocol Reference: Section 9. 5

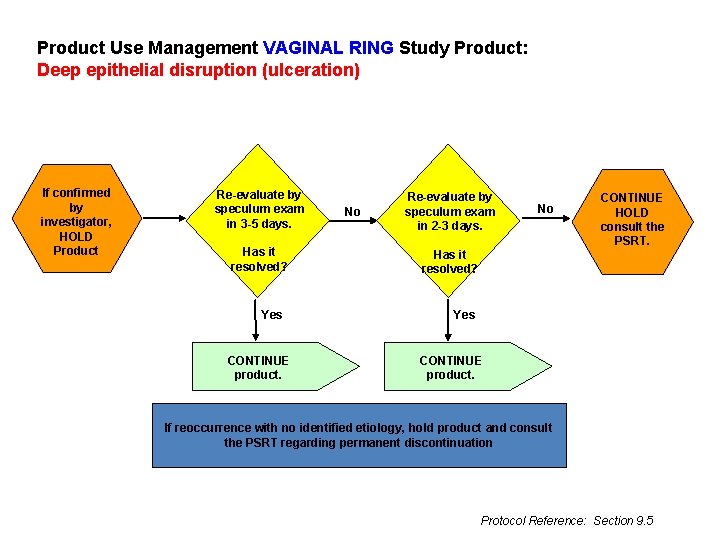
Product Use Management VAGINAL RING Study Product: Deep epithelial disruption (ulceration) If confirmed by investigator, HOLD Product Re-evaluate by speculum exam in 3 -5 days. Has it resolved? Yes CONTINUE product. No Re-evaluate by speculum exam in 2 -3 days. No CONTINUE HOLD consult the PSRT. Has it resolved? Yes CONTINUE product. If reoccurrence with no identified etiology, hold product and consult the PSRT regarding permanent discontinuation Protocol Reference: Section 9. 5

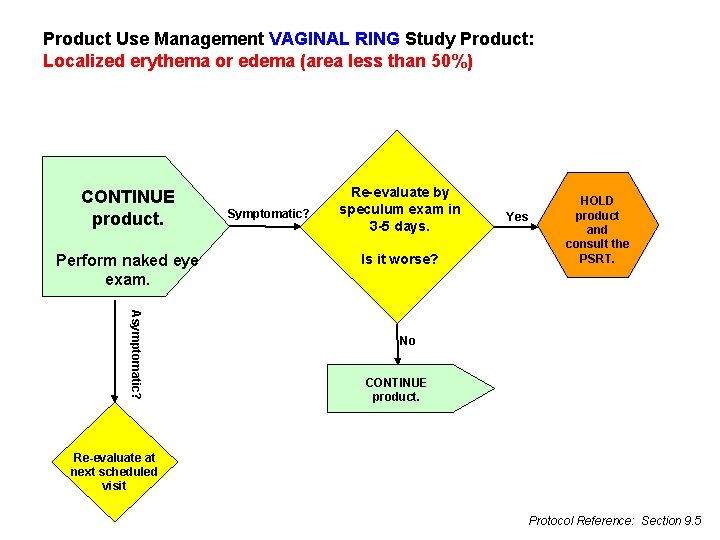
Product Use Management VAGINAL RING Study Product: Localized erythema or edema (area less than 50%) CONTINUE product. Perform naked eye exam. Symptomatic? Re-evaluate by speculum exam in 3 -5 days. Is it worse? Yes HOLD product and consult the PSRT. Asymptomatic? No CONTINUE product. Re-evaluate at next scheduled visit Protocol Reference: Section 9. 5

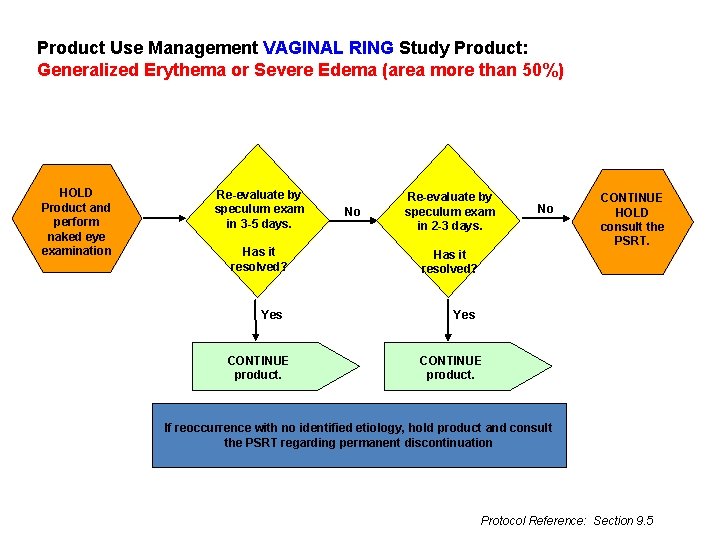
Product Use Management VAGINAL RING Study Product: Generalized Erythema or Severe Edema (area more than 50%) HOLD Product and perform naked eye examination Re-evaluate by speculum exam in 3 -5 days. Has it resolved? Yes CONTINUE product. No Re-evaluate by speculum exam in 2 -3 days. No CONTINUE HOLD consult the PSRT. Has it resolved? Yes CONTINUE product. If reoccurrence with no identified etiology, hold product and consult the PSRT regarding permanent discontinuation Protocol Reference: Section 9. 5

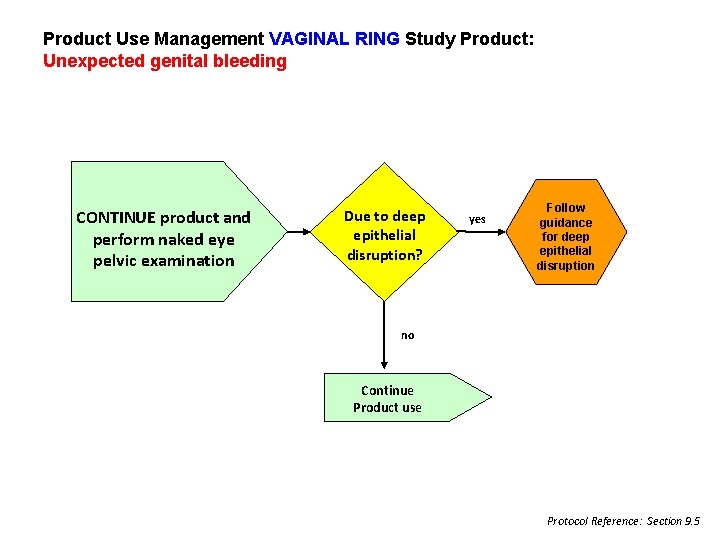
Product Use Management VAGINAL RING Study Product: Unexpected genital bleeding CONTINUE product and perform naked eye pelvic examination Due to deep epithelial disruption? yes Follow guidance for deep epithelial disruption no Continue Product use Protocol Reference: Section 9. 5

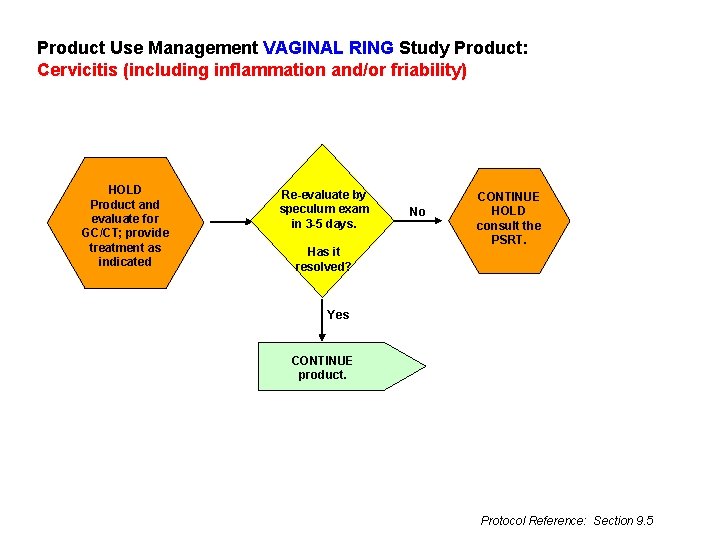
Product Use Management VAGINAL RING Study Product: Cervicitis (including inflammation and/or friability) HOLD Product and evaluate for GC/CT; provide treatment as indicated Re-evaluate by speculum exam in 3 -5 days. Has it resolved? No CONTINUE HOLD consult the PSRT. Yes CONTINUE product. Protocol Reference: Section 9. 5

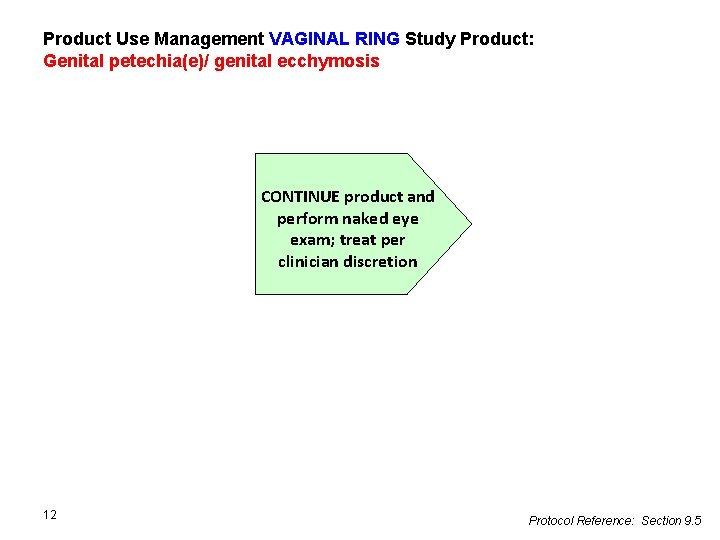
Product Use Management VAGINAL RING Study Product: Genital petechia(e)/ genital ecchymosis CONTINUE product and perform naked eye exam; treat per clinician discretion 12 Protocol Reference: Section 9. 5