Primary and secondary structural organization of proteins Commissionerate







- Slides: 11

Primary and secondary structural organization of proteins Commissionerate of Collegiate Education

• The four important biomolecules carbohydrates, proteins, lipids and nucleic acids. • Most abundant • 50 % dry weight of cell Commissionerate of Collegiate Education

• All proteins are made of only 20 types of amino acids. • A protein molecule is a polymer of amino acids. • Before we study the structure of proteins let us study the structure of amino acid. Commissionerate of Collegiate Education

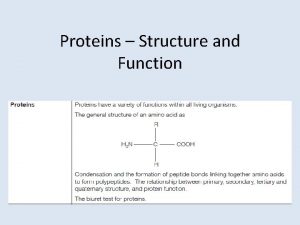
General structure of amino acid • In the middle of the amino acid molecule a central carbon atom • It is called α carbon atom. • An acidic carboxyl – COOH and, a basic – NH 2 group • As well as a H atom are attached to the α carbon atom. Commissionerate of Collegiate Education

• Only the 4 th position changes among the amino acids. • It is called the “R” group. • It is this group that gives specificity to an amino acid. Commissionerate of Collegiate Education

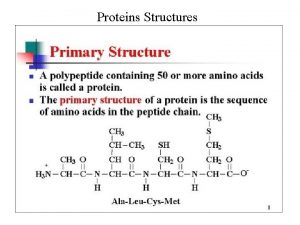
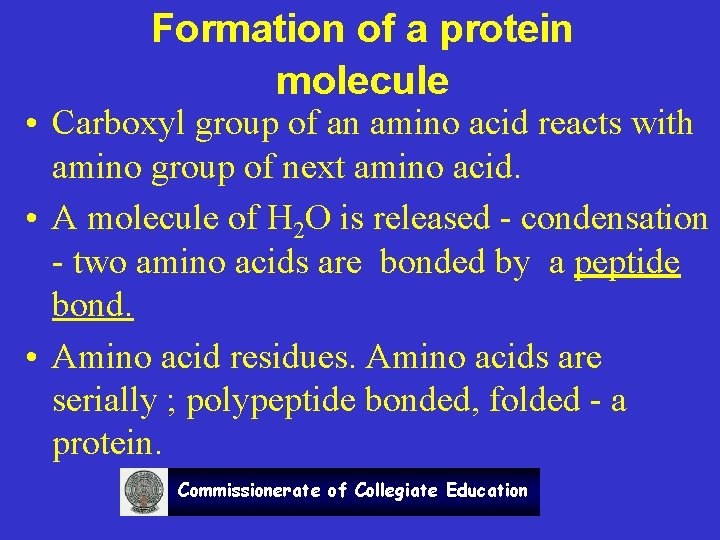
Formation of a protein molecule • Carboxyl group of an amino acid reacts with amino group of next amino acid. • A molecule of H 2 O is released - condensation - two amino acids are bonded by a peptide bond. • Amino acid residues. Amino acids are serially ; polypeptide bonded, folded - a protein. Commissionerate of Collegiate Education

• 4 levels of structural organization • • 1 Primary structure 2 Secondary structure 3 Tertiary structure and 4 Quaternary structure Commissionerate of Collegiate Education

• 1 Primary structure • Exact sequence of amino acids • Lysozyme; 129 residues seuence is primary structure of lysozyme • 4 disulphide bonds. • Free amino group on left side, free carboxl group on right side. Commissionerate of Collegiate Education

• Insulin; A chain-21 residues • B chain 30 residues; sequence of A chain, B chain primary strucrure of insulin. 2 disulphide bonds. • Human body- thousands of types • Substitution of even one amino acid-serious consequences. Commissionerate of Collegiate Education

2. Secondary sturcture • 3 dimensional form of all sections of back bone. Two forms • 1. α helix and 2. β pleated sheet. • α helix; common, pulled spiral spring; maintained by H bonds between adjacent CO and NH groups; 1 st to 5 th : 3. 6 ; keratin Commissionerate of Collegiate Education

• β pleated sheet; less common • Fibroin-many polypeptide chains; parallel; same direction or antiparallel • C=O and NH groups, and NH and C=O groups of adjacent polypeptide chain joined by H bonds • All C=O and NH groups H bonded, strong, stable. Commissionerate of Collegiate Education
 Primary secondary tertiary quaternary
Primary secondary tertiary quaternary Commissionerate of industries
Commissionerate of industries Structural proteins function
Structural proteins function Function of muscular tissue
Function of muscular tissue Structural role of proteins
Structural role of proteins Globular proteins examples
Globular proteins examples Levels of structural organization
Levels of structural organization Structural frame of an organization
Structural frame of an organization The human body an orientation
The human body an orientation Organizational structure of the pnp
Organizational structure of the pnp Point by point organization example
Point by point organization example Primary and secondary effects of a tectonic hazard
Primary and secondary effects of a tectonic hazard