PLAY GAME trial Post Concussion in Youth Randomized







- Slides: 15

PLAY GAME trial: Post Concussion in Youth Randomized, placebo-controlled trial of Melatonin Dr. Karen M. Barlow Associate Professor of Pediatric Neurology Director of the Alberta Children’s Hospital TBI Research Program

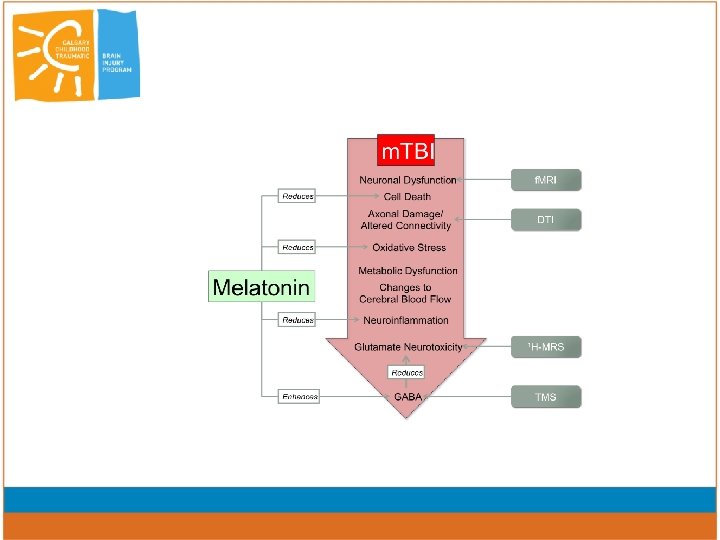
Melatonin Mechanisms of action • lipophilic and crosses BBB easily • receptor-mediated (physiological levels) – MT 1 and MT 2 – retinoid orphan nuclear hormone receptors • cell differentiation and immune response • non-receptor mediated (physiological and supraphysiological) – broad spectrum, direct free radical scavenger and antioxidant

Melatonin: Mechanisms of action • Neuroprotective • GABAergic effects • Modulates neuroinflammation • Pain • Anxiety • Sleep

Melatonin in TBI models • decreases brain edema, BBB permeability and ICP - inhibition of oxidative stress (Kabadi 2010; Dehghan 2013) • Increased antioxidant enzymes, and in particular lowering lipid peroxidation (Kerman 2005; Beni 2004, Ozdemir 2005) • decreases oxidative stress, inactivation of transcriptional factors (cytokine signaling) (Tsai 2011) • prevented tau hyperphosphorylation (Gutierrez-Cuesta 2007) • MEL and dexamethasone reduction in lesion size less apoptosis (Campolo 2013); • MEL and MIN – no effect (Kelso, 2011)

• Melatonin has been registered in 203 trials with clinicaltrials. gov • 75 trials ongoing – – – HIE Stroke IUGR Acute MI Oncology

Safe No significant complications Does not suppress endogenous production Dose? – – Speculative 3 mg saturates receptors Non-receptor mediated require higher levels Neonatal studies 10 mg/kg/dose over 2 hours with repeated dosing over 72 hours.

Neonatal brain injury • Open label term neonates with sepsis • decreased mortality • RCT in HIE term neonates – decreased mortality • Two RCTs in preterm – decreased mortality (total 230 patients) Ongoing trials: • PREMELIP trial – neuroprotective effects (white matter) in very preterm • Phase II trial – RCT – post-natal melatonin in preterm infants and brain injury

Melatonin in paediatric TBI • 18 children with m. TBI, headaches and sleep disorder • Time since injury was > 10. 6 months post-injury • Treatment response was assessed every 4 weeks. • Treatment response: significant decrease in headaches of >50% was considered a response. • 7 children 3 -5 mg MEL; 11 children 6 -10 mg MEL • Response to melatonin 81% – All treatments 64%

PLAY GAME trial: PCS in Youth and the GABAergic effects of Melatonin • Randomized double blind placebo controlled trial – Dose dependent response assessment – parallel group design • Placebo • 3 mg melatonin • 10 mg melatonin • Use adjunctive techniques (integrating f. MRI, DTI and TMS) to elucidate alterations in neuronal function and circuitry (including Cortical Excitation)


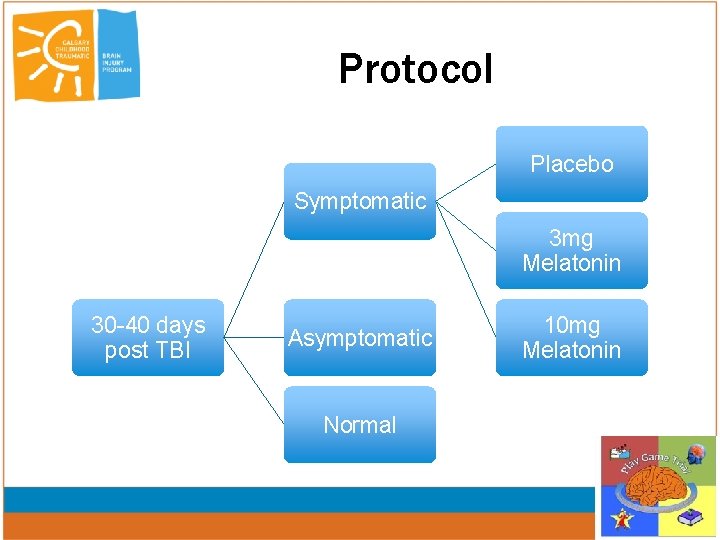
Protocol Placebo Symptomatic 3 mg Melatonin 30 -40 days post TBI Asymptomatic Normal 10 mg Melatonin

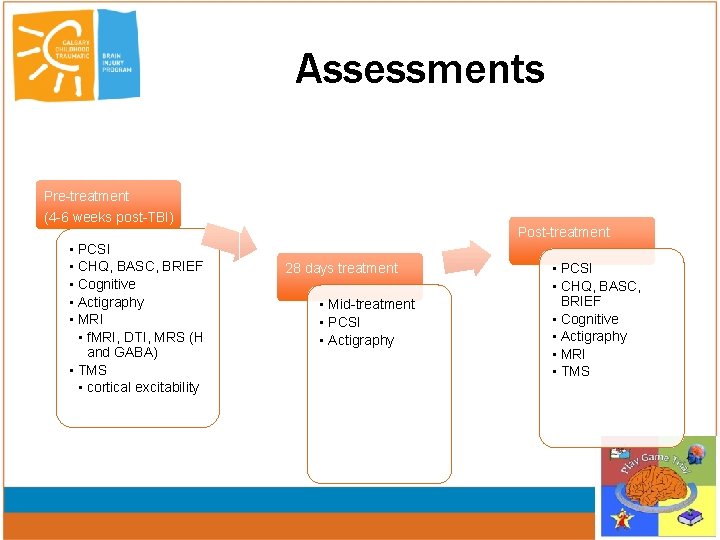
Assessments Pre-treatment (4 -6 weeks post-TBI) • PCSI • CHQ, BASC, BRIEF • Cognitive • Actigraphy • MRI • f. MRI, DTI, MRS (H and GABA) • TMS • cortical excitability Post-treatment 28 days treatment • Mid-treatment • PCSI • Actigraphy • PCSI • CHQ, BASC, BRIEF • Cognitive • Actigraphy • MRI • TMS

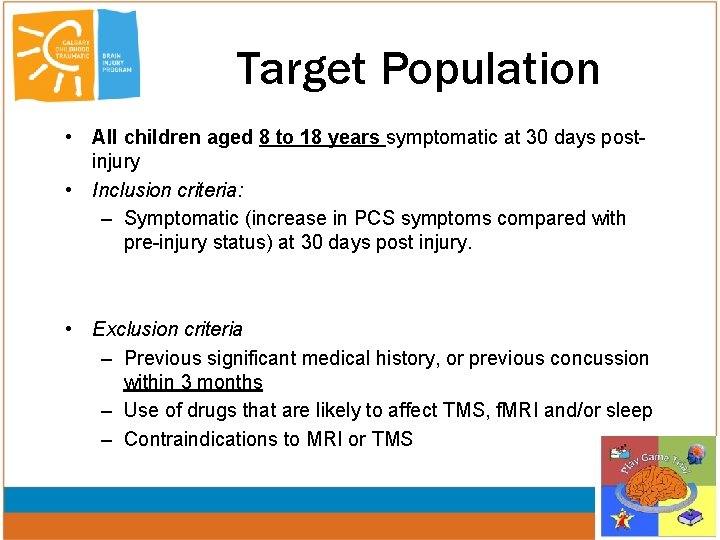
Target Population • All children aged 8 to 18 years symptomatic at 30 days postinjury • Inclusion criteria: – Symptomatic (increase in PCS symptoms compared with pre-injury status) at 30 days post injury. • Exclusion criteria – Previous significant medical history, or previous concussion within 3 months – Use of drugs that are likely to affect TMS, f. MRI and/or sleep – Contraindications to MRI or TMS

Trial Progress – Trial documentation, registration and committees established – Health Canada/Ethical approval August 2013 – 5 p study November 2013 – Recruitment started December 2013 – Bi-monthly Trial Steering Committee meetings – DSMB met April 2013 – Slow recruitment • 13 participants to date » No withdrawals or adverse events • HCT-Amendment to double potential participants – – Age range increased 8 – 18 years If Previous concussion > 3 months prior

Acknowledgements www. playgametrial. ca • • Collaborators Deborah Dewey – Co-PI Brenda Turley – Trial coordinator • Lisette Lockyer • • • Brian Brooks – Neuropsychology Adam Kirton – TMS Val Kirk – Sleep dysfunction Frank Mac. Master – f. MRI/MRS Michael Esser – Translational models Alberto Nettel-Aguirre – Biostatistics Susan Crawford – Biostatistics Jeff Buchhalter – Medical advisor Angelo Mikrogianakis – ED medicine David Johnson – Clinical trials Roger Zemek – Ottawa site • Carolyn Emery (independent advisor) DSMB • • • Jamie Hutcheson Robert Platt Lawrence Richer Post-grad Students • Trevor Seegor • Angela Villavincencio-Requis