Physics I Chap 15 Fluid Statics Prof WAN


![Categorization I F Fluids [Latin, to flow]: liquids & gases – Flow to take Categorization I F Fluids [Latin, to flow]: liquids & gases – Flow to take](https://slidetodoc.com/presentation_image_h2/dfe5af3666c91b2d2344a4c5664dd24c/image-5.jpg)









- Slides: 37

Physics I Chap 15. Fluid Statics Prof. WAN, Xin xinwan@zimp. zju. edu. cn http: //zimp. zju. edu. cn/~xinwan/

Motivation F Already learned: – Single-particle mechanics: translational and rotational variables – Two particles: center of mass + relative motion – Many particles: center of mass only F Collective phenomena: liquids, solids 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 2

Definitions F State of matter: phases – Gases: e. g. , water vapor, oxygen – Liquids: e. g. , water, blood, mercury – Solids: e. g. , ice, glacier, rock, rubber “… physicist rejects the definition of a solid as (roughly) what hurts your toe when you kick it …” -- Concepts in Solids, by P. W. Anderson 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 3

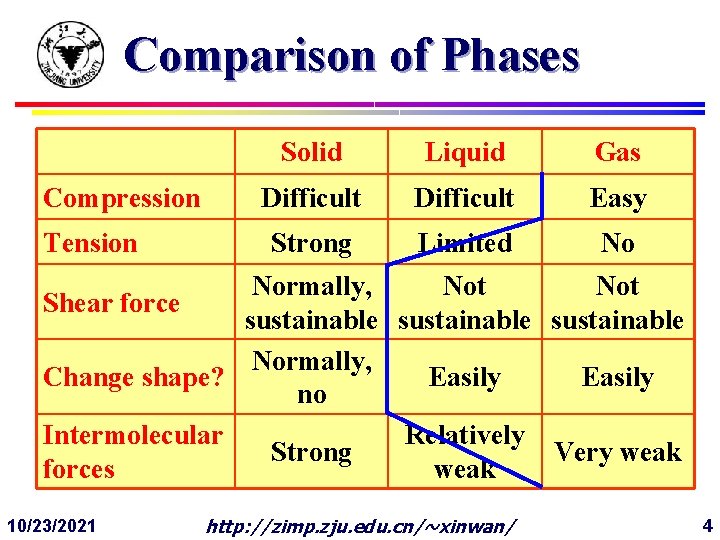
Comparison of Phases Compression Tension Solid Liquid Gas Difficult Easy Strong Limited No Normally, Not Shear force sustainable Normally, Change shape? Easily no Intermolecular Relatively Strong Very weak forces weak 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 4
![Categorization I F Fluids Latin to flow liquids gases Flow to take Categorization I F Fluids [Latin, to flow]: liquids & gases – Flow to take](https://slidetodoc.com/presentation_image_h2/dfe5af3666c91b2d2344a4c5664dd24c/image-5.jpg)
Categorization I F Fluids [Latin, to flow]: liquids & gases – Flow to take the shape of container F Normally, solids do not flow F Ch. 15. Statics: fluids at rest Ch. 16. Dynamics: fluids in motion F 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 5

Categorization II F Condensed matter: solids & liquids – (Almost) incompressible – Density changes little with temperature (at constant pressure) F Gases – Easily compressible – Density changes substantially with temperature (at constant pressure) 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 6

Modern Notions F F Gases: full symmetry -- disordered Solids: reduced symmetry -- ordered – Think about snowflakes (hexagonal) F F Liquids: short-range order only Phase transitions – Disorder to order: symmetry breaking – Pioneered by Landau, Anderson, … 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 7

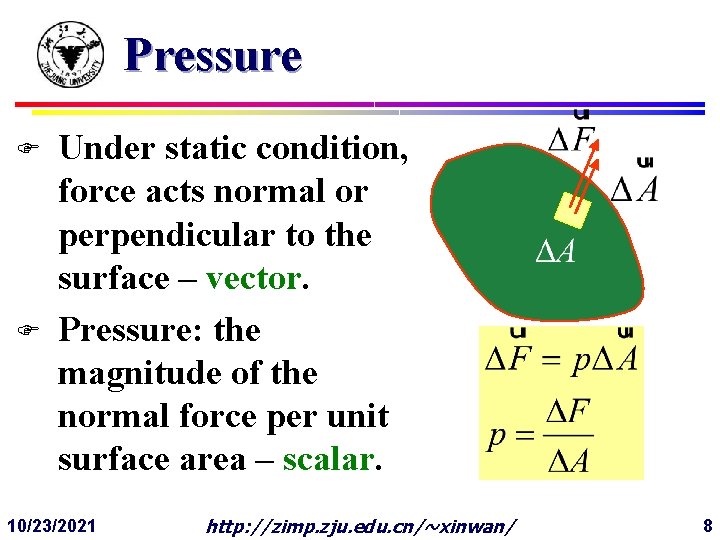
Pressure F F Under static condition, force acts normal or perpendicular to the surface – vector. Pressure: the magnitude of the normal force per unit surface area – scalar. 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 8

Common Units 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 9

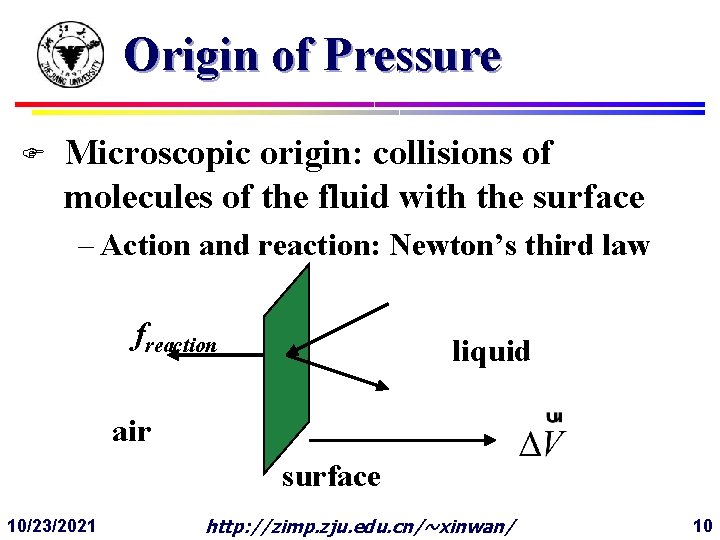
Origin of Pressure F Microscopic origin: collisions of molecules of the fluid with the surface – Action and reaction: Newton’s third law freaction liquid air surface 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 10

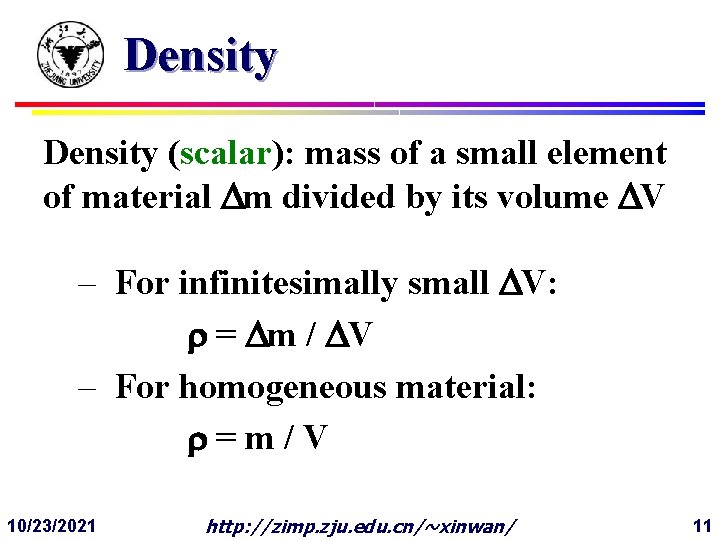
Density (scalar): mass of a small element of material Dm divided by its volume DV – For infinitesimally small DV: = Dm / DV – For homogeneous material: =m/V 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 11

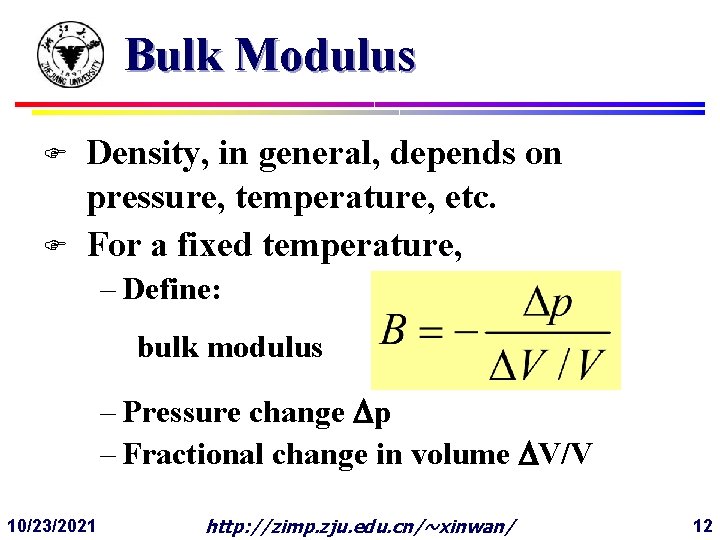
Bulk Modulus F F Density, in general, depends on pressure, temperature, etc. For a fixed temperature, – Define: bulk modulus – Pressure change Dp – Fractional change in volume DV/V 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 12

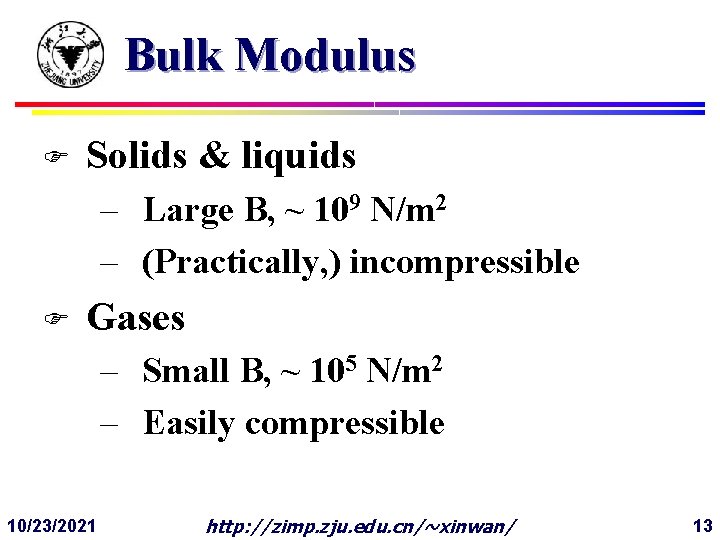
Bulk Modulus F Solids & liquids – Large B, ~ 109 N/m 2 – (Practically, ) incompressible F Gases – Small B, ~ 105 N/m 2 – Easily compressible 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 13

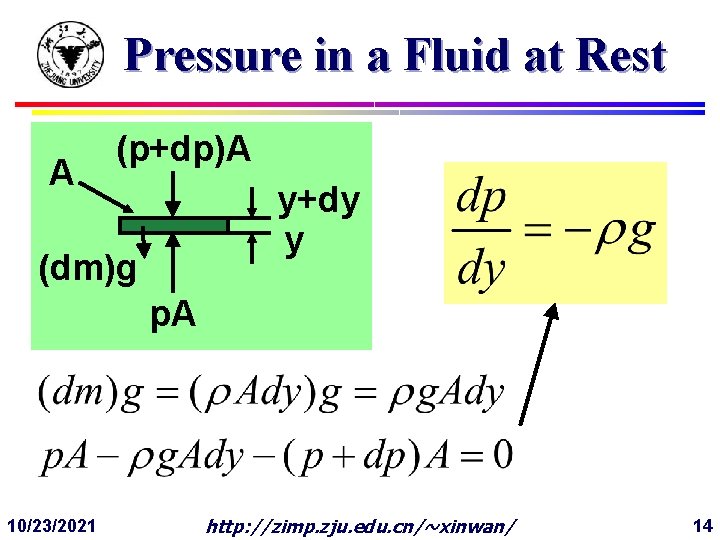
Pressure in a Fluid at Rest A (p+dp)A y+dy y (dm)g p. A 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 14

Pressure in a Fluid at Rest F Assumption: , g independent of y 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 15

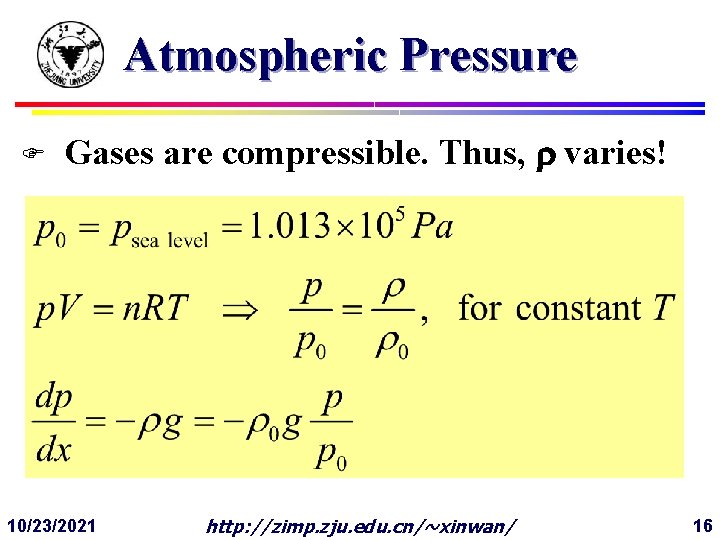
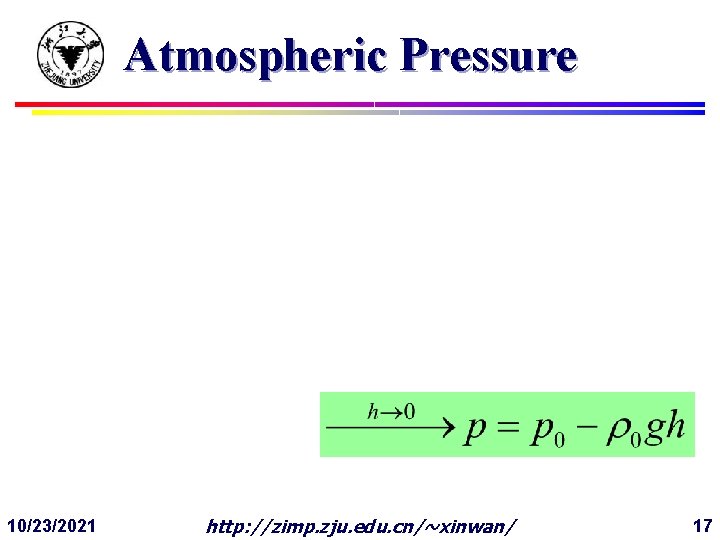
Atmospheric Pressure F Gases are compressible. Thus, varies! 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 16

Atmospheric Pressure 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 17

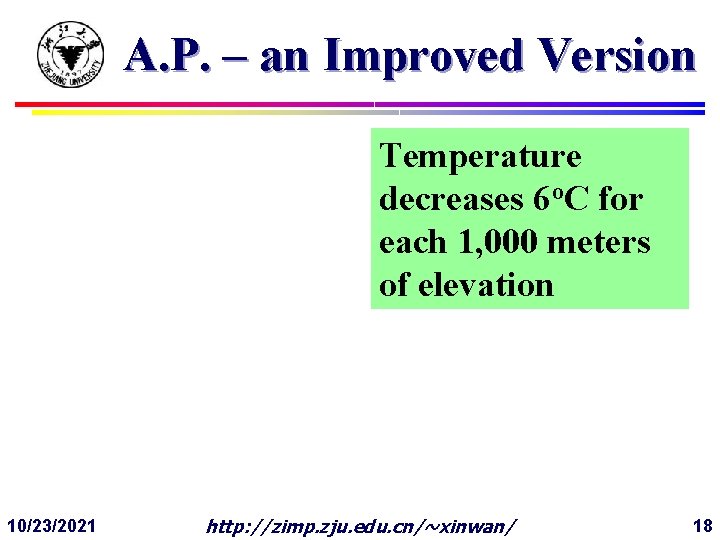
A. P. – an Improved Version Temperature decreases 6 o. C for each 1, 000 meters of elevation 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 18

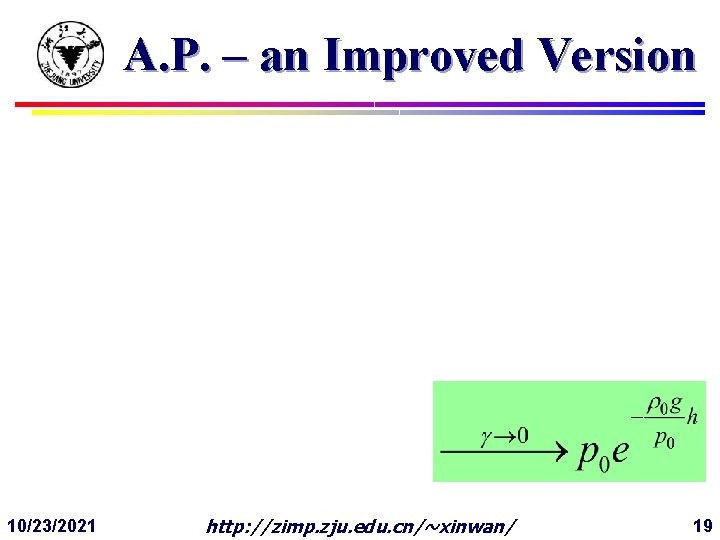
A. P. – an Improved Version 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 19

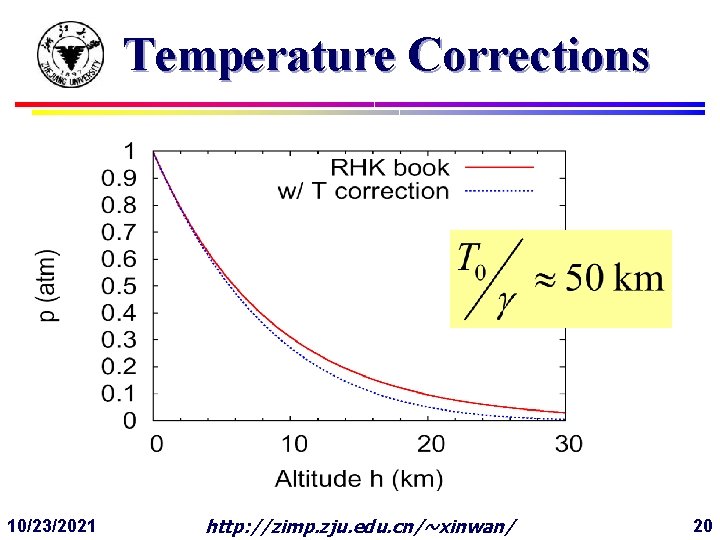
Temperature Corrections 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 20

Blaise Pascal (1623 -62) French mathematician & philosopher; founder of modern theory of probability; discovered Pascal's law of pressure and principle of the hydraulic press. 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 21

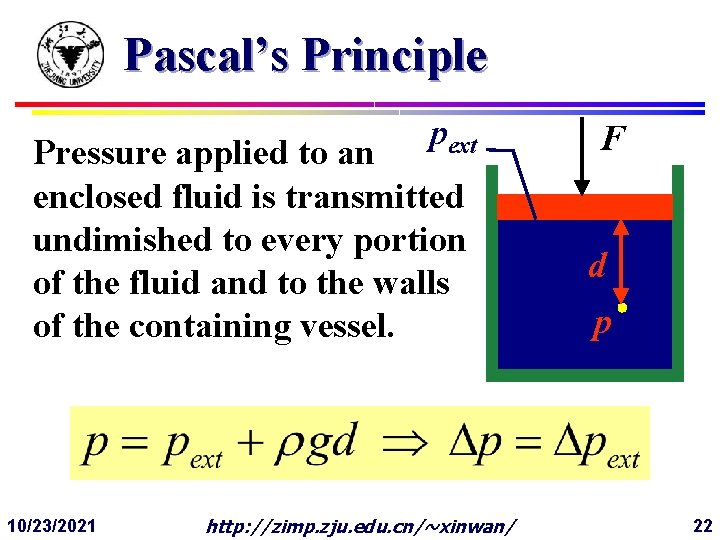
Pascal’s Principle pext Pressure applied to an enclosed fluid is transmitted undimished to every portion of the fluid and to the walls of the containing vessel. 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ F d p 22

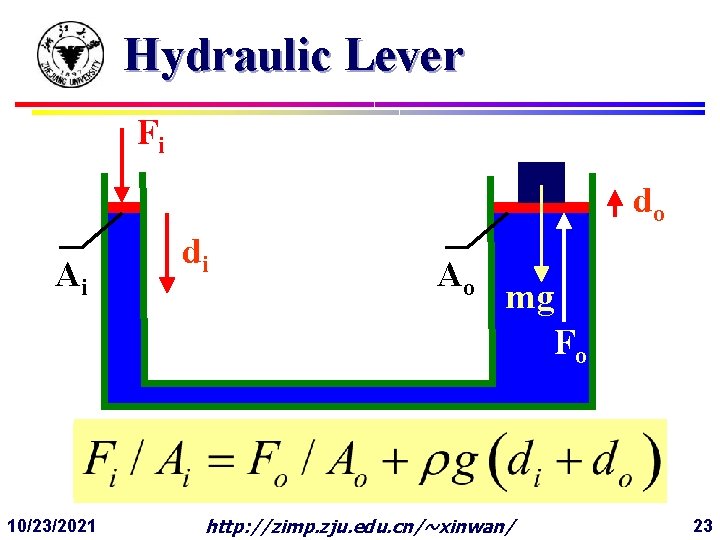
Hydraulic Lever Fi do Ai 10/23/2021 di Ao mg Fo http: //zimp. zju. edu. cn/~xinwan/ 23

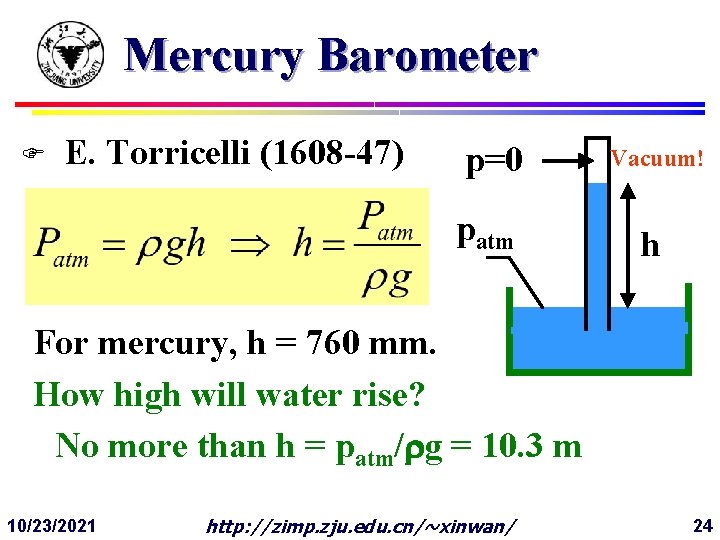
Mercury Barometer F E. Torricelli (1608 -47) p=0 patm Vacuum! h For mercury, h = 760 mm. How high will water rise? No more than h = patm/ g = 10. 3 m 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 24

Q & A: On Pressure Q: Is it possible to stand on the roof of a five-story (20 m) building and drink, using a straw, from a glass on the ground? 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 20 m 25

Q & A on Pressure A: Even if a person could completely remove all of 20 m the air from the straw, the height to which the outside air pressure pushes the water up the straw would not be high enough for him/her to drink the water, no matter how hard he/she sucks! 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 26

Archimedes of Syracuse Archimedes (circa 287 -212 B. C. ) Greek mathematician & inventor known especially for work in mechanics; discovered principle of buoyancy; wrote on volumes of spheres and cylinders, value of p, etc. 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 27

Archimedes of Syracuse Give me a place to stand I will move the earth. 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 28

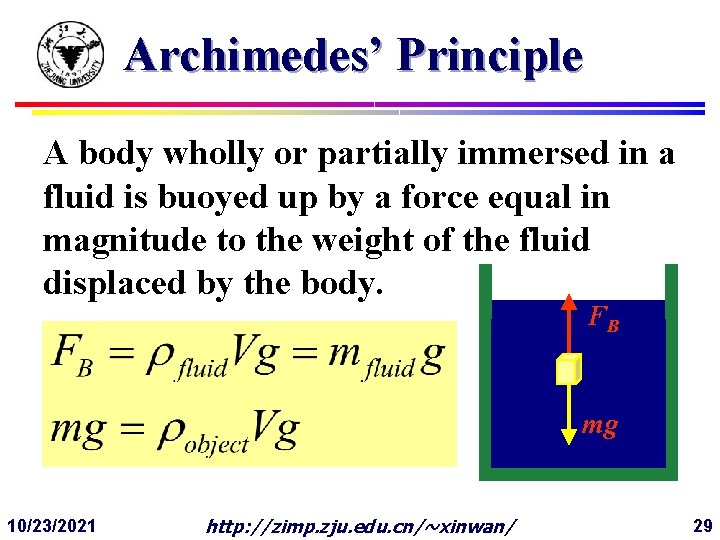
Archimedes’ Principle A body wholly or partially immersed in a fluid is buoyed up by a force equal in magnitude to the weight of the fluid displaced by the body. FB mg 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 29

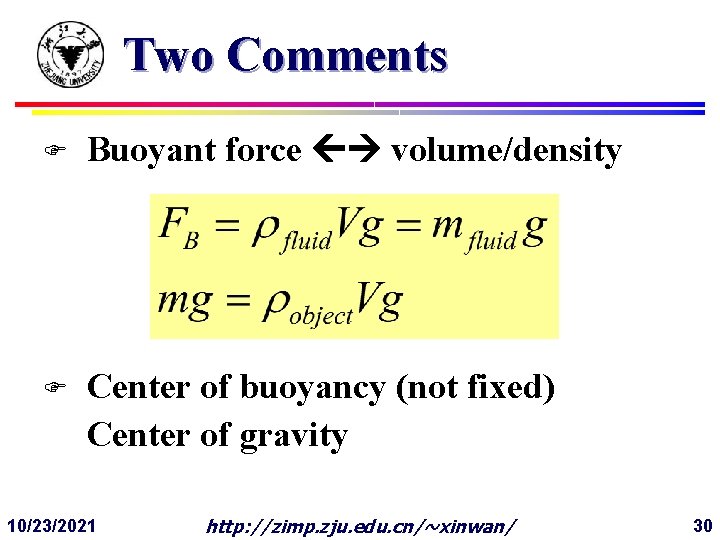
Two Comments F Buoyant force volume/density F Center of buoyancy (not fixed) Center of gravity 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 30

Q & A: Insect on Water Q: Consider an insect of mass m = 2 x 10 kg with feet of radius r = 1. 5 x 10 -4 m walking on water. Find the contact angle f. How much weight can the insect put on before it falls in the water? -5 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 31

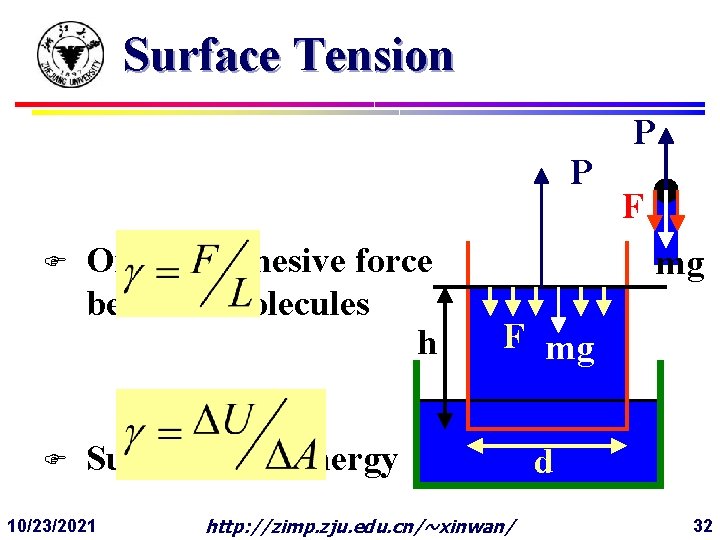
Surface Tension P F F Origin: Cohesive force between molecules h F mg Surface costs energy 10/23/2021 P http: //zimp. zju. edu. cn/~xinwan/ d 32

Q & A: Insect on Water A: The weight of the insect is mg and this must be supported by the upward force of surface tension, so that, 6 * (2 pr) * g cos(f) = mg f o Solving yields, f = 62. The maximum weight that can be supported is 6 * 2 pr g = 3. 4 x 10 -5 kg 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 33

Take-home Question F Can you invent a scale (or some other mechanical devices) that one can use to read the percentage of silver in the gold crown (let’s say, to help Archimedes to determine the exact percentage) without explicit calculations? 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 34

Eureka 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 35

Summary F F F Definitions and categorizations Variables: pressure and density Equations and principles – Variation of pressure in a fluid at rest – Pascal’s principle – Archimedes’s principle of buoyancy F Hydraulic lever; mercury barometer 10/23/2021 http: //zimp. zju. edu. cn/~xinwan/ 36

Homework Fluid Statics: Pages 348 -349 Problems: 4, 14 10/23/2021 Fluid Dynamics: Page 369 Problems: 4, 14 http: //zimp. zju. edu. cn/~xinwan/ 37