Fluids Statics Level 1 Physics Essential Questions and






- Slides: 19

Fluids - Statics Level 1 Physics

Essential Questions and Objectives Essential Questions Objectives What are the physical properties of Define atmospheric pressure, gauge pressure and absolute pressure and the relationship among these terms Define and apply the concept of fluid pressure State and apply Archimedes' principle to calculate the buoyant force Demonstrate proficiency in accurately drawing and labeling free-body diagrams involving buoyant force and other forces State the characteristics of an ideal fluid Understand that Bernoulli's equation is a statement of conservation of energy Demonstrate proficiency in solving problems involving changes in depth and/or changes in pressure and/or changes in velocity fluid states of matter? What is pressure? Is the term “suction” real? What is an ideal fluid? What happens to velocity when the area of an opening inc/dec? State and apply Pascal's principle in practical situations such as hydraulic lifts Apply the equation of continuity in solving problems

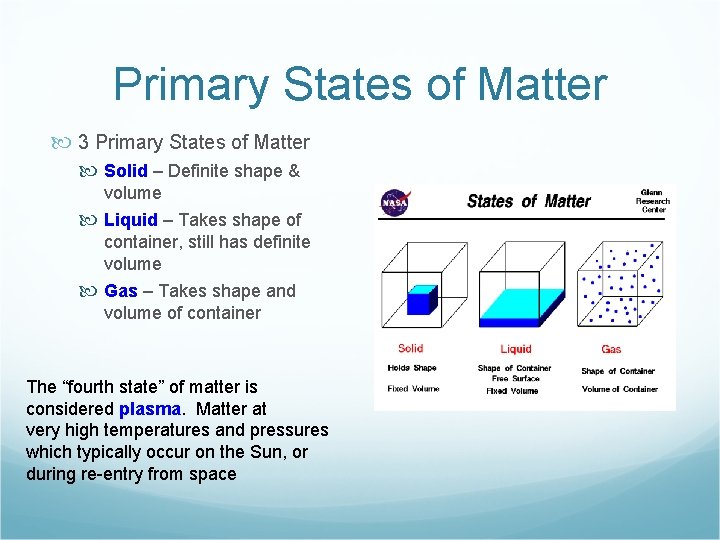
Primary States of Matter 3 Primary States of Matter Solid – Definite shape & volume Liquid – Takes shape of container, still has definite volume Gas – Takes shape and volume of container The “fourth state” of matter is considered plasma. Matter at very high temperatures and pressures which typically occur on the Sun, or during re-entry from space

Density is an important factor that determines the behavior of a fluid Density is the mass per unit volume of a substance. The variable given to density is the Greek letter ρ

Pressure Why should we be concerned about fluids? Pressure is transmitted through liquid. Since liquid is effectively incompressible, pressure applied to a liquid is transmitted without loss throughout the liquid.

Pressure An important application in fluids would be the pressure of a fluid. Pressure is defined as the amount of force per unit area

Example A water bed is 2. 0 m on a side an 30. 0 cm deep. a. Find its weight if the density of water is 1000 kg/m 3. b. Find the pressure that the water bed exerts on the floor. Assume that the entire lower surface of the bed makes contact with the floor. 1. 2 m 3 1200 kg 11760 N 2940 N/m 2

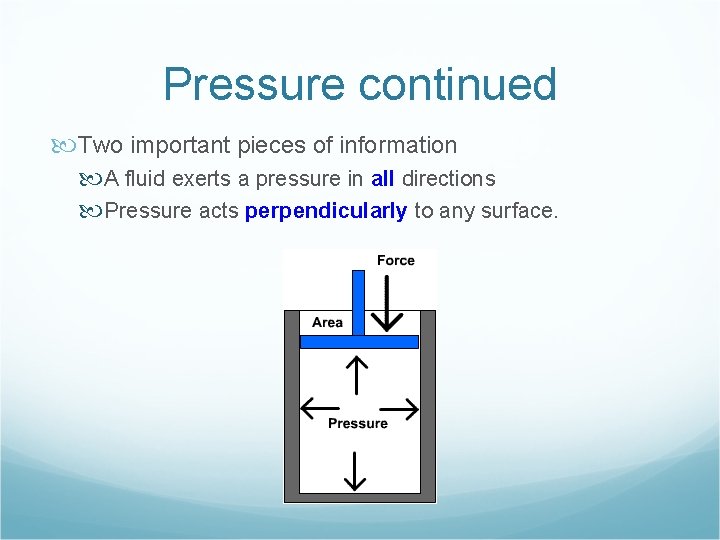
Pressure continued Two important pieces of information A fluid exerts a pressure in all directions Pressure acts perpendicularly to any surface.

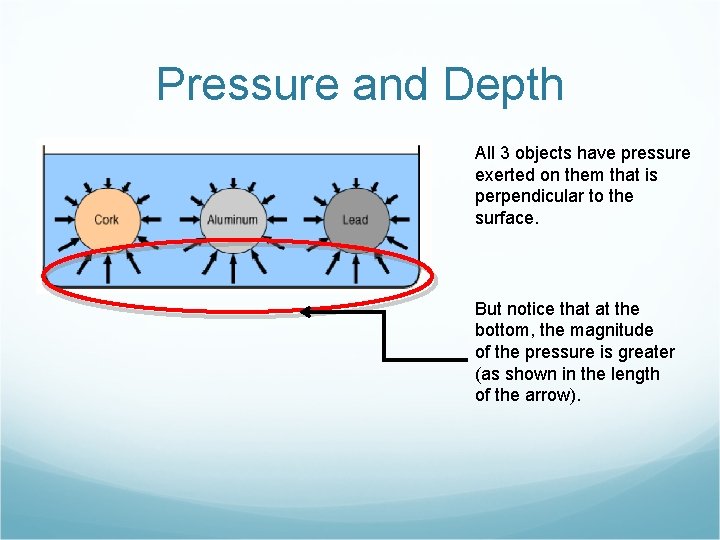
Pressure and Depth All 3 objects have pressure exerted on them that is perpendicular to the surface. But notice that at the bottom, the magnitude of the pressure is greater (as shown in the length of the arrow).

Pressure vs. Depth – Submerged Object Fatm An object is submerged just under the surface. In the FBD at the right, what are the 3 forces acting on the object? 1. The weight (mg) the object 2. The force of the atmosphere pressing down (Fatm) mg Fwater 3. The force of the water pushing up (Fwater)

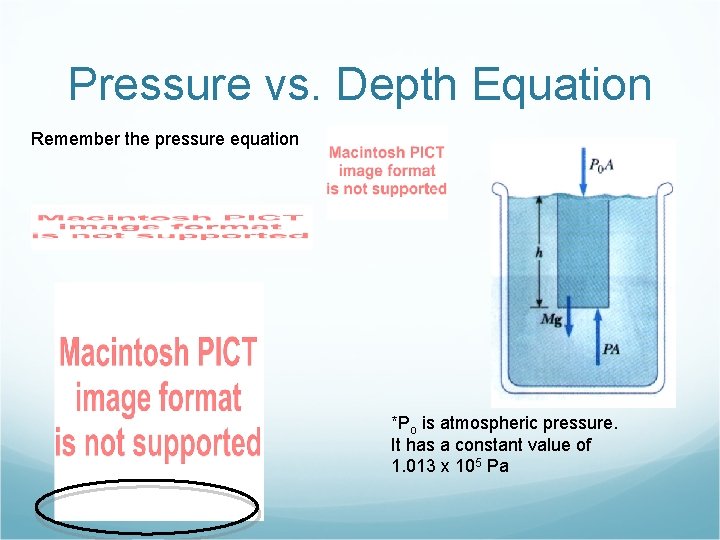
Pressure vs. Depth Equation Remember the pressure equation *Po is atmospheric pressure. It has a constant value of 1. 013 x 105 Pa

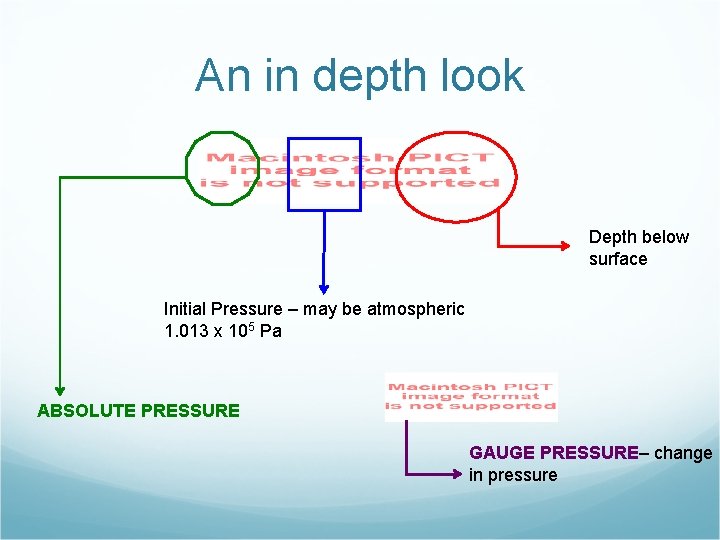
An in depth look Depth below surface Initial Pressure – may be atmospheric 1. 013 x 105 Pa ABSOLUTE PRESSURE GAUGE PRESSURE– change in pressure

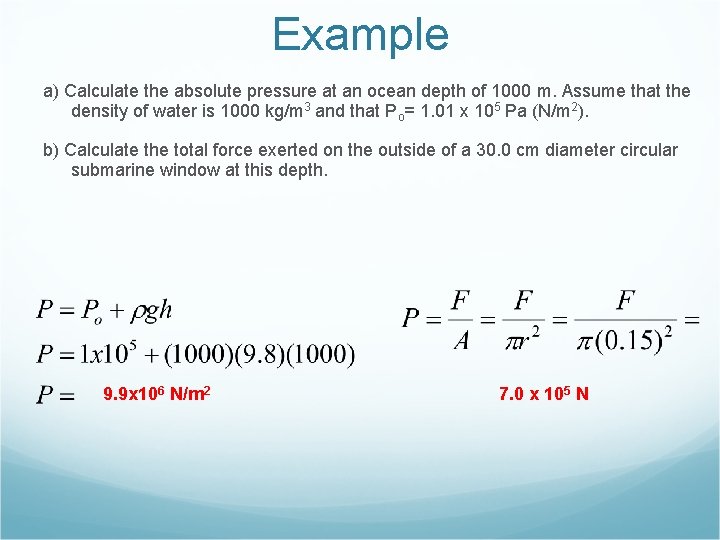
Example a) Calculate the absolute pressure at an ocean depth of 1000 m. Assume that the density of water is 1000 kg/m 3 and that Po= 1. 01 x 105 Pa (N/m 2). b) Calculate the total force exerted on the outside of a 30. 0 cm diameter circular submarine window at this depth. 9. 9 x 106 N/m 2 7. 0 x 105 N

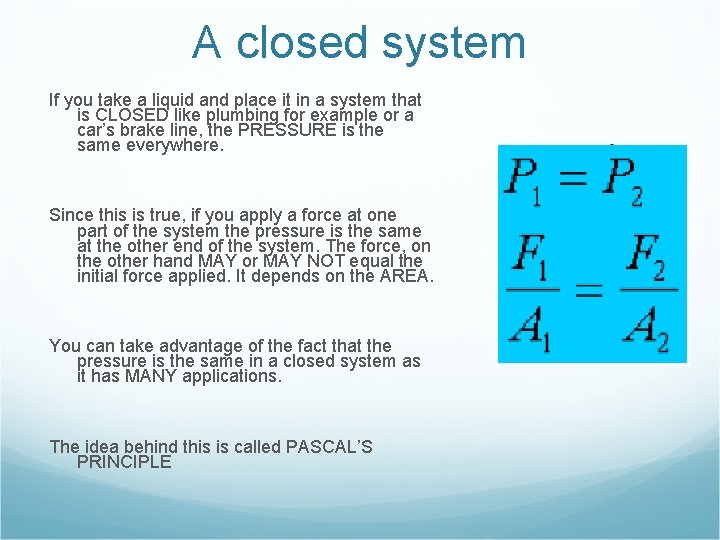
A closed system If you take a liquid and place it in a system that is CLOSED like plumbing for example or a car’s brake line, the PRESSURE is the same everywhere. Since this is true, if you apply a force at one part of the system the pressure is the same at the other end of the system. The force, on the other hand MAY or MAY NOT equal the initial force applied. It depends on the AREA. You can take advantage of the fact that the pressure is the same in a closed system as it has MANY applications. The idea behind this is called PASCAL’S PRINCIPLE

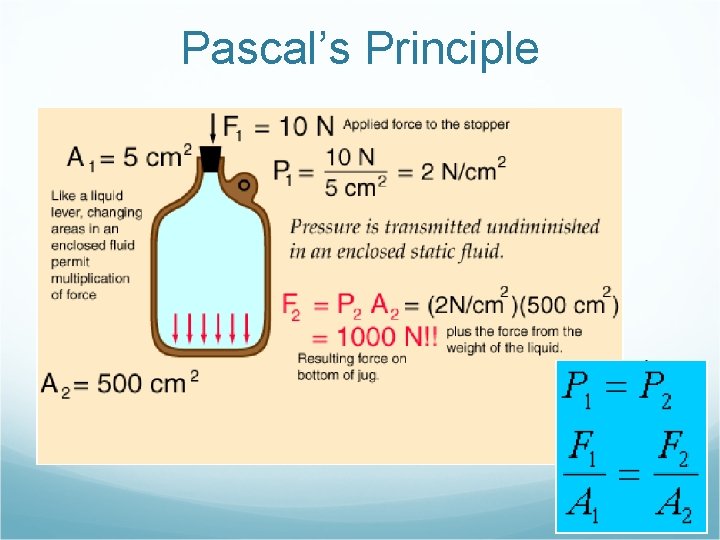
Pascal’s Principle

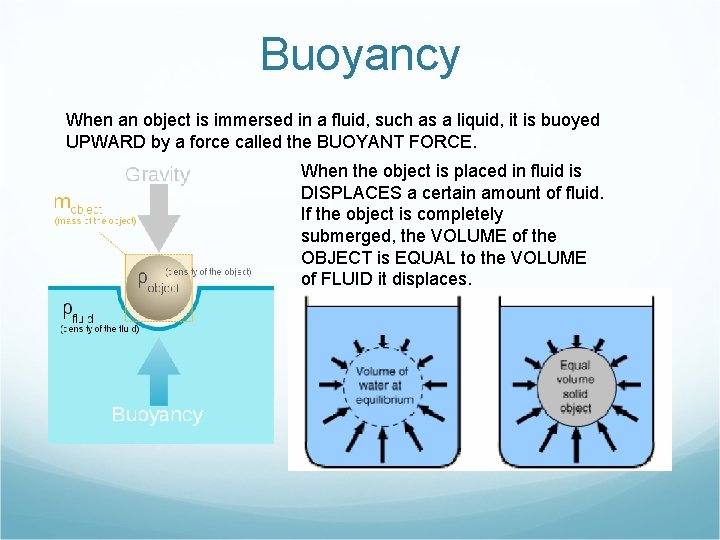
Buoyancy When an object is immersed in a fluid, such as a liquid, it is buoyed UPWARD by a force called the BUOYANT FORCE. When the object is placed in fluid is DISPLACES a certain amount of fluid. If the object is completely submerged, the VOLUME of the OBJECT is EQUAL to the VOLUME of FLUID it displaces.

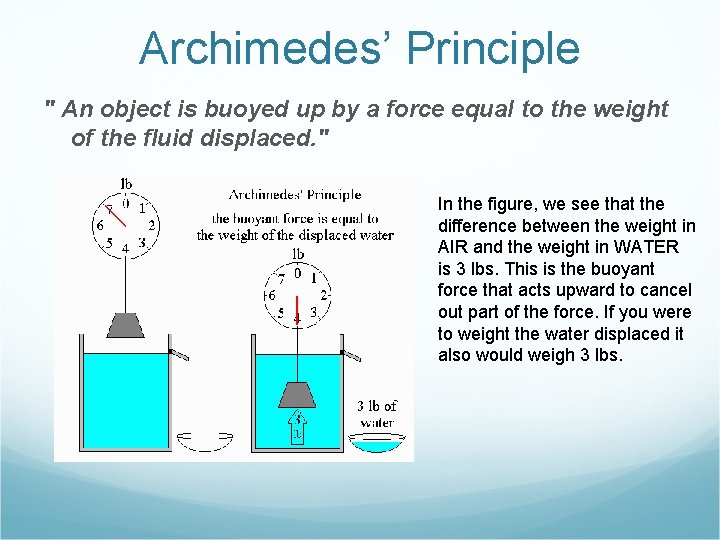
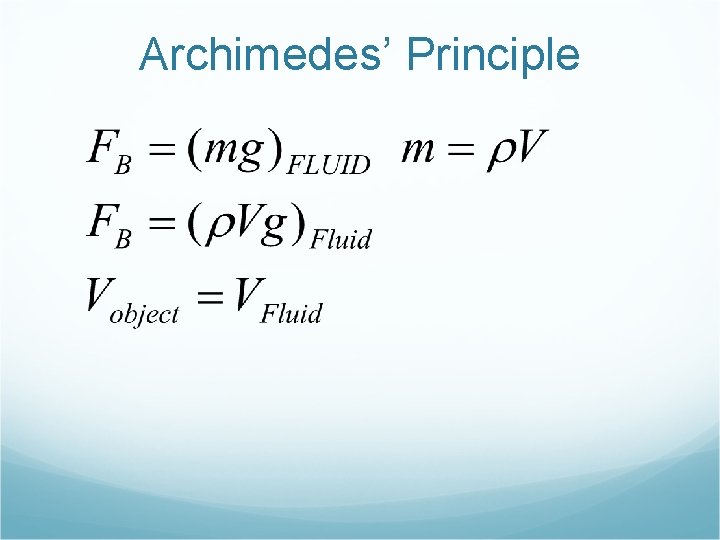
Archimedes’ Principle " An object is buoyed up by a force equal to the weight of the fluid displaced. " In the figure, we see that the difference between the weight in AIR and the weight in WATER is 3 lbs. This is the buoyant force that acts upward to cancel out part of the force. If you were to weight the water displaced it also would weigh 3 lbs.

Archimedes’ Principle

Example A bargain hunter purchases a "gold" crown at a flea market. After she gets home, she hangs it from a scale and finds its weight in air to be 7. 84 N. She then weighs the crown while it is immersed in water (density of water is 1000 kg/m 3) and now the scale reads 6. 86 N. Is the crown made of pure gold if the density of gold is 19. 3 x 103 kg/m 3? 0. 98 N NO! This is NOT gold as 8000<19300 0. 0001 m 3 0. 80 kg 8000 kg/m 3