Phosphorus Announcements Proposals Last weeks question of the























- Slides: 36

Phosphorus Announcements: Proposals

Last week's question of the day Two lakes with the same surface area and similar levels of P & N are located within km of each other, but one routinely experiences fish kills, and the other does not. What characteristics of these lakes are likely influencing this pattern? The winterkill lake is shallow enough that the water does not contain enough oxygen to sustain oxic conditions during ice cover.

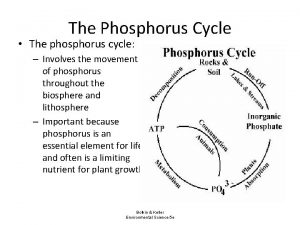
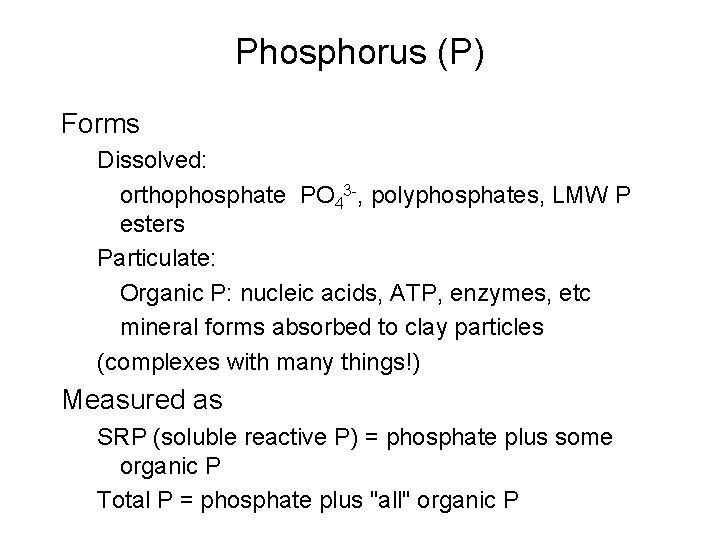
Phosphorus (P) Forms Dissolved: orthophosphate PO 43 -, polyphosphates, LMW P esters Particulate: Organic P: nucleic acids, ATP, enzymes, etc mineral forms absorbed to clay particles (complexes with many things!) Measured as SRP (soluble reactive P) = phosphate plus some organic P Total P = phosphate plus "all" organic P

Phosphorus Unlike N, P does not directly partake in redox rxns, although availability is regulated, in part, by redox reactions. Often the limiting nutrient in lake systems (but not always true for riverine systems)

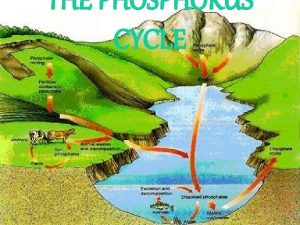
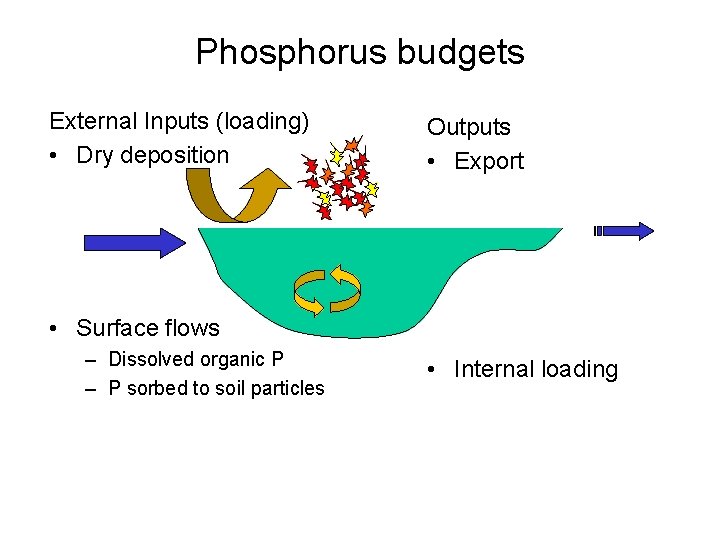
Phosphorus budgets External Inputs (loading) • Dry deposition Outputs • Export • Surface flows – Dissolved organic P – P sorbed to soil particles • Internal loading

Watershed contributions to P inputs • Little P is transported to lakes if watershed is well-watered and vegetated (P is in high demand) • Lots of P is transported to lake in poorlyvegetated areas (including ag)

Watershed contributions, con't • Dominant bedrock in area determines overall P availability • (e. g. igneous rock has low P)

Internal P-cycling views over time • Classical theory – Proposed by Einsele & Ohle, Germany, and Mortimer, England, early 1940's • Sulfur modifications – Proposed by (Hasler and Einsele 1948) • Modern theory - the role of bacteria

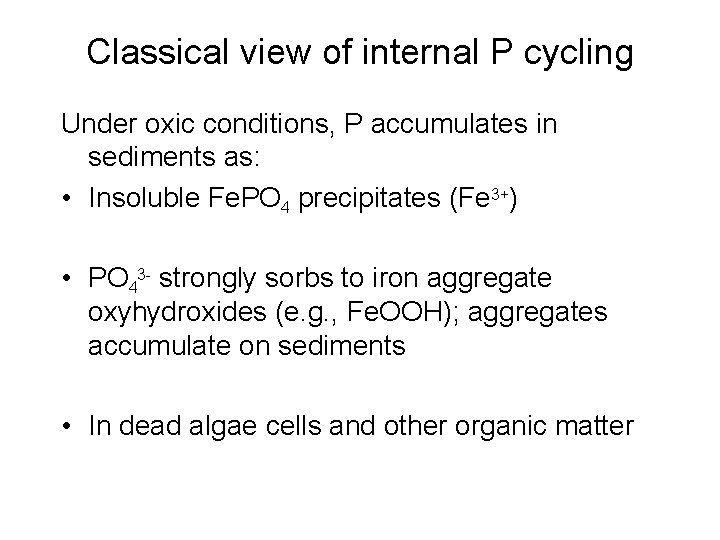
Classical view of internal P cycling Under oxic conditions, P accumulates in sediments as: • Insoluble Fe. PO 4 precipitates (Fe 3+) • PO 43 - strongly sorbs to iron aggregate oxyhydroxides (e. g. , Fe. OOH); aggregates accumulate on sediments • In dead algae cells and other organic matter

Classical view - anoxic conditions Internal Loading = Sediment or hypolimnetic release of P • In anoxic conditions, Fe. OOH-PO 4 and Fe. PO 4 complexes dissolve, releasing PO 43 - and Fe 2+ • Sediment PO 43 - concentrations 5 -20 times greater than water column • If water column remains oxic, Fe 3+ precipitates and aggregates on the sediment surface prevent released PO 43 - from diffusing upwards from anoxic sediments and/or entering the water column

The P-cycling model, updated The role of sulfur • Microbial reduction of SO 42 - yields S 2 • Sulfide forms Fe. S or Fe. S 2 (insoluble) • If enough Fe is removed, less P-Fe complexes are formed and more P remains available Sulfur uptake of Fe not important in lakes with low levels of S (e. g. , igneous rock watershed)

Modern model of P-cycling Classical models assumed that microbes indirectly affected P cycling by utilizing dissolved O 2, NO 3 -, SO 42 -, Fe 3+ and Mn 4+ as electron donors and there by affecting the solubility of chemical species versus Modern models suggest that microbes play an active part in P-cycling

Why re-evaluate? • Fe 2+ and PO 43 - were not released simultaneously as they should if process were completely chemical • Observed that sediments less able to take up P when sterilized with antibiotics, implying bacterial role • In some lakes, P is not released when the hypolimnion becomes anoxic, suggesting that sediment P content and retention is not controlled only by O 2

Roles of bacteria in P-cycling • Bacteria release P during decomposition – SRP directly into water column following cell lysis – polyphosphate granules accumulated under aerobic conditions Important because between 10 and 75 % of potentially soluble sediment P in microbes • Iron reducing bacteria (use Fe 3+ as electron acceptor) are necessary to solubilize the Fe-P aggregates under anoxic conditions

Other processes of P release • Elevated p. H – may replace P absorbed to Fe. OOH flocs with OH- • Benthic algae films – may reduce P-release while photosynthesizing, and increase P-release while respiring • Turbulence (wind or gas bubbles) – Allows dissolved P in anoxic sediments to bypass Fe floc layer and pass directly to water column

Other processes of P release • Bioturbation – Introduces O 2 into sediments – In the process of resuspending sediments, release soluble P • Rooted aquatic plants – Release P that originated from sediments www. fishontario. com/articles/ carp-european-style/

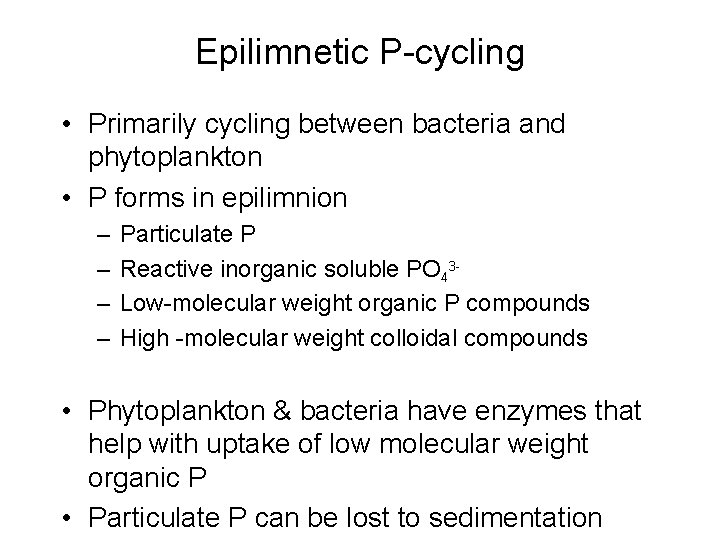
Epilimnetic P-cycling • Primarily cycling between bacteria and phytoplankton • P forms in epilimnion – – Particulate P Reactive inorganic soluble PO 43 Low-molecular weight organic P compounds High -molecular weight colloidal compounds • Phytoplankton & bacteria have enzymes that help with uptake of low molecular weight organic P • Particulate P can be lost to sedimentation

Are lakes P-sinks or sources? • Depends on lake characteristics…

Lakes with oxic hypolimnia • Usually have what type of characteristics? Large hypolimnia that holds large mass of O 2 during stratification Low productivity

Lakes with oxic hypolimnia • P sink – Store about 2 x as much external P load than lakes with anoxic hypolimnia – P stored in sediments increases exponentially with water residence time (WRT) • Retained P = 1/(1+sqrt(WRT)) • Deep lakes with WRT > 25 yrs often retain 70 -90 % P input permanently in sediments

Lakes with anoxic hypolimnia • Usually have what types of characteristics? Relatively small hypolimnia Short(er) water residence times High external P load High productivity

Lakes with anoxic hypolimnia • P-source • Experience high internal P loading – Significant amounts of P is not stored permanently in sediments

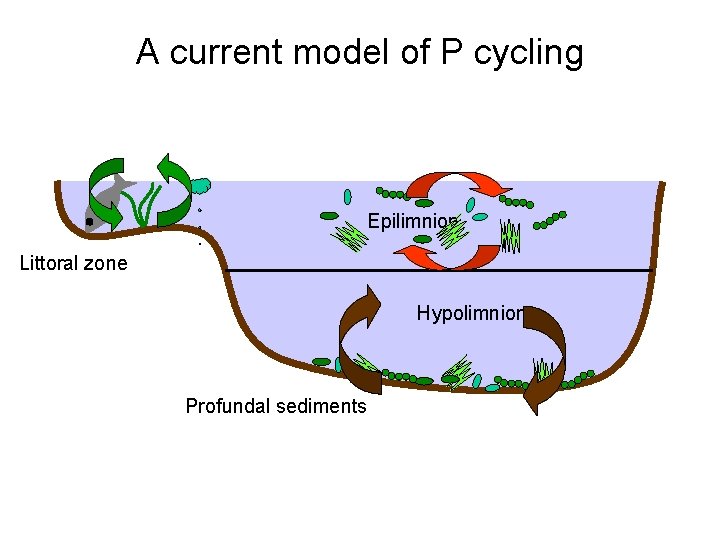
A current model of P cycling Epilimnion Littoral zone Hypolimnion Profundal sediments

Anthropogenic eutrophication

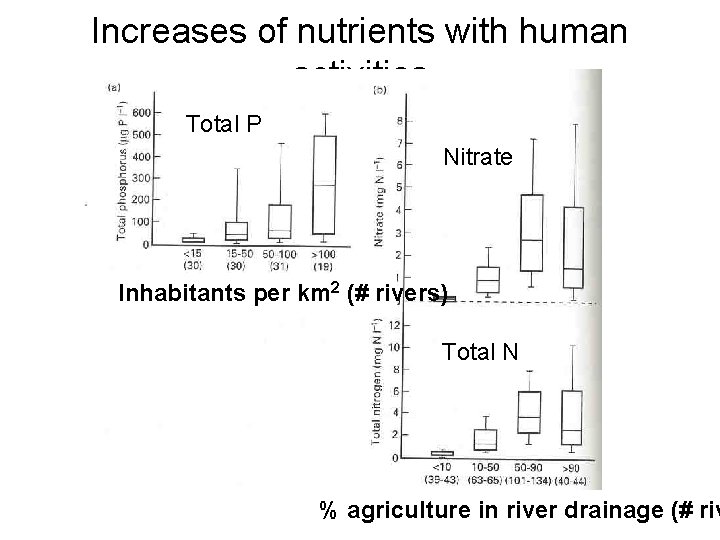
Increases of nutrients with human activities Total P Nitrate Inhabitants per km 2 (# rivers) Total N % agriculture in river drainage (# riv

The detergent wars 1969 -1970 Detergent foam from a fountain in front of the National Gallery of Art, Wash. , DC, in 1959, when nonbiodegradable detergents were in common use. Under gov't pressure, the detergent

But detergents still contained phosporus… • In 1969, greater than %50 of phosphorus in municipal waster was from detergents (in the form of polyphosphates) • Huge increase since 1949 in the powdered detergents used in washing machines • Role of P in detergents was primarily to soften water • Powerful U. S. Soap & Detergent Industry strongly resisted change

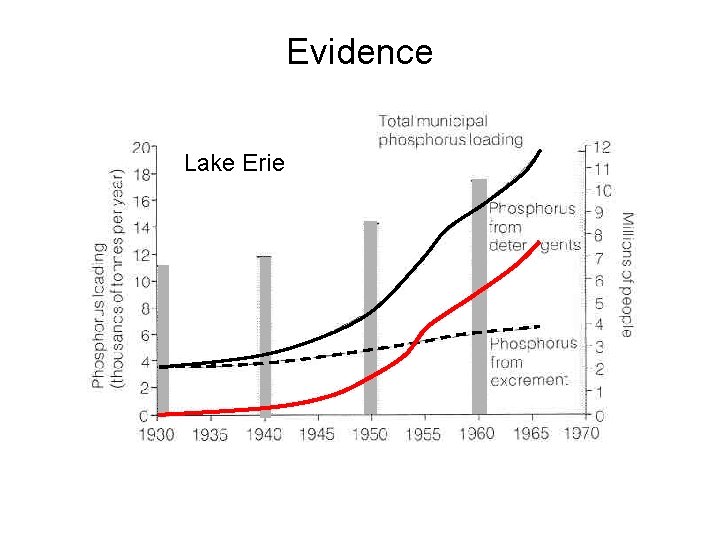
Evidence Lake Erie

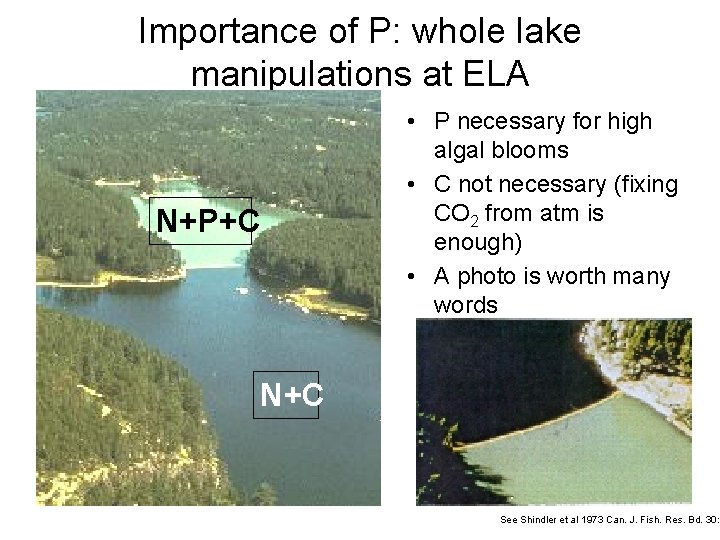
Importance of P: whole lake manipulations at ELA N+P+C • P necessary for high algal blooms • C not necessary (fixing CO 2 from atm is enough) • A photo is worth many words N+C See Shindler et al 1973 Can. J. Fish. Res. Bd. 30: 1

• My power detergent now says: "biodegradable anionic and nonionic surfactants (followed by lots of unspecified ingredients)…. Contains less than 0. 5% phosphorus by weight"

P remediations

Lake Washington, Seattle, WA

Lake Washington wastewater diversion Chlorophy ll-a 1967 nutrient diversion completed Total P 1963 nutrient diversion begun (~28%) See Edmondson and Lehman 1981 Limnology and

Lake Washington wastewater diversion • One of the first U. S. studies to demonstrate the feasibility and impact of reducing secondary and primary effluent sources • Succeeded in part due to Lake Washington's – very deep basin that during stratification remained oxygenated, even at the peak of eutrophication – rapid flushing rate (short WRT) – primarily urban and forested watershed

Other methods of remediation • • Point source reductions (Lake Washington) Sewage treatment plants Diversions Use natural or constructed wetlands to absorb nutrients • Buffer strips • Precipitate water column P Add aluminum sulfate (alum) or ferric chloride to precipitate P as Al. PO 4, Fe. PO 4 or Fe(OOH)PO 4 • Dredge P-rich sediments • Withdraw hypolimnetic water that is high in dissolved P

Question of the day: • Explain why Lake Washington's watershed, morphology and flushing rate influenced recovery from nutrient loading. WHY are these characteristics important? • Under what conditions (lake characteristics) would simply reducing P-inputs not work? Why not?
 Costa's level of questions
Costa's level of questions Pvu announcement
Pvu announcement Kayl announcements
Kayl announcements /r/announcements
/r/announcements General announcements
General announcements David ritthaler
David ritthaler Page 113 of fahrenheit 451
Page 113 of fahrenheit 451 Prefatory elements in proposal exclude
Prefatory elements in proposal exclude What is solicited external
What is solicited external Delhi muslim proposals
Delhi muslim proposals Writing and completing reports and proposals
Writing and completing reports and proposals Nature of fire insurance contract
Nature of fire insurance contract Formal business report
Formal business report Developing effective research proposals
Developing effective research proposals When evaluating cost-cutting proposals
When evaluating cost-cutting proposals Title of the study
Title of the study Artificial intelligence thesis proposals
Artificial intelligence thesis proposals Eclipse computing proposals slow
Eclipse computing proposals slow Gestational age in weeks
Gestational age in weeks Bma bank holiday lieu days
Bma bank holiday lieu days Weeks of supply formula
Weeks of supply formula How many weeks
How many weeks Where is the embryo located
Where is the embryo located Four weeks prior to christmas
Four weeks prior to christmas Shannon weeks
Shannon weeks This weeks lesson
This weeks lesson Complete each space with one word
Complete each space with one word Antenatal investigations
Antenatal investigations 3rd 9 weeks exam review chemistry
3rd 9 weeks exam review chemistry 4 weeks before christmas
4 weeks before christmas Two week notice letter example
Two week notice letter example What is amniotic fluid
What is amniotic fluid Rhyming words with mystery
Rhyming words with mystery Mems microfluidics
Mems microfluidics According to walter pauk, 10 weeks after lecture
According to walter pauk, 10 weeks after lecture Two weeks have passed since the new moon
Two weeks have passed since the new moon Dr veronica white
Dr veronica white