Phase 3 Treatment Nave and Treatment Experienced HIV



- Slides: 9

Phase 3 Treatment Naïve and Treatment Experienced HIV Coinfection Simeprevir in HIV Coinfection, GT-1 C 212 Trial Dieterich D, et al. Clin Infect Dis. 2014; 59: 1579 -87. Hepatitis web study

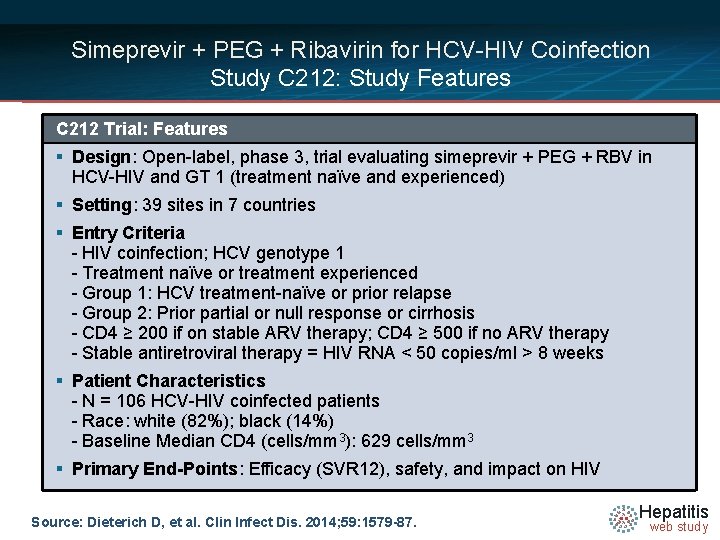
Simeprevir + PEG + Ribavirin for HCV-HIV Coinfection Study C 212: Study Features C 212 Trial: Features § Design: Open-label, phase 3, trial evaluating simeprevir + PEG + RBV in HCV-HIV and GT 1 (treatment naïve and experienced) § Setting: 39 sites in 7 countries § Entry Criteria - HIV coinfection; HCV genotype 1 - Treatment naïve or treatment experienced - Group 1: HCV treatment-naïve or prior relapse - Group 2: Prior partial or null response or cirrhosis - CD 4 ≥ 200 if on stable ARV therapy; CD 4 ≥ 500 if no ARV therapy - Stable antiretroviral therapy = HIV RNA < 50 copies/ml > 8 weeks § Patient Characteristics - N = 106 HCV-HIV coinfected patients - Race: white (82%); black (14%) - Baseline Median CD 4 (cells/mm 3): 629 cells/mm 3 § Primary End-Points: Efficacy (SVR 12), safety, and impact on HIV Source: Dieterich D, et al. Clin Infect Dis. 2014; 59: 1579 -87. Hepatitis web study

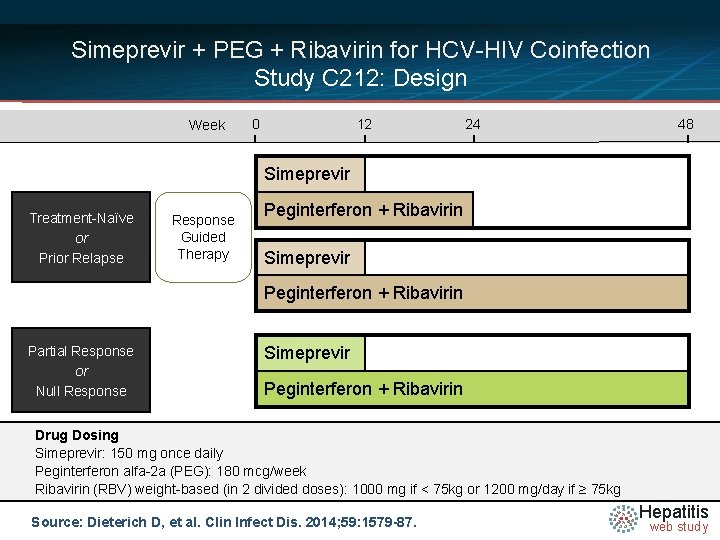
Simeprevir + PEG + Ribavirin for HCV-HIV Coinfection Study C 212: Design Week 0 12 24 48 Simeprevir Treatment-Naïve or Prior Relapse Response Guided Therapy Peginterferon + Ribavirin Simeprevir Peginterferon + Ribavirin Partial Response or Null Response Simeprevir Peginterferon + Ribavirin Drug Dosing Simeprevir: 150 mg once daily Peginterferon alfa-2 a (PEG): 180 mcg/week Ribavirin (RBV) weight-based (in 2 divided doses): 1000 mg if < 75 kg or 1200 mg/day if ≥ 75 kg Source: Dieterich D, et al. Clin Infect Dis. 2014; 59: 1579 -87. Hepatitis web study

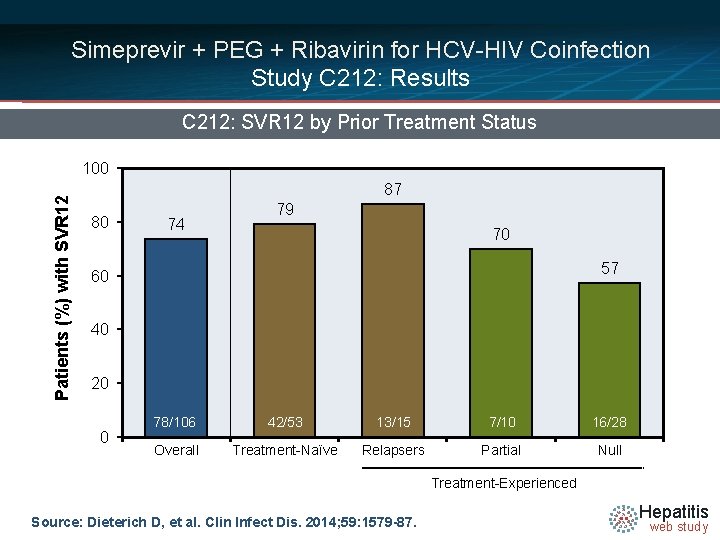
Simeprevir + PEG + Ribavirin for HCV-HIV Coinfection Study C 212: Results C 212: SVR 12 by Prior Treatment Status Patients (%) with SVR 12 100 87 80 74 79 70 57 60 40 20 0 78/106 42/53 13/15 7/10 16/28 Overall Treatment-Naïve Relapsers Partial Null Treatment-Experienced Source: Dieterich D, et al. Clin Infect Dis. 2014; 59: 1579 -87. Hepatitis web study

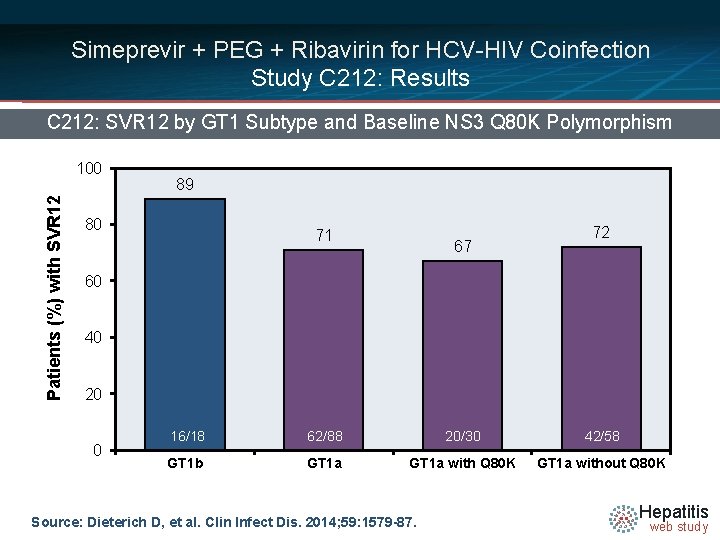
Simeprevir + PEG + Ribavirin for HCV-HIV Coinfection Study C 212: Results C 212: SVR 12 by GT 1 Subtype and Baseline NS 3 Q 80 K Polymorphism Patients (%) with SVR 12 100 89 80 71 67 72 60 40 20 0 16/18 62/88 20/30 42/58 GT 1 b GT 1 a with Q 80 K GT 1 a without Q 80 K Source: Dieterich D, et al. Clin Infect Dis. 2014; 59: 1579 -87. Hepatitis web study

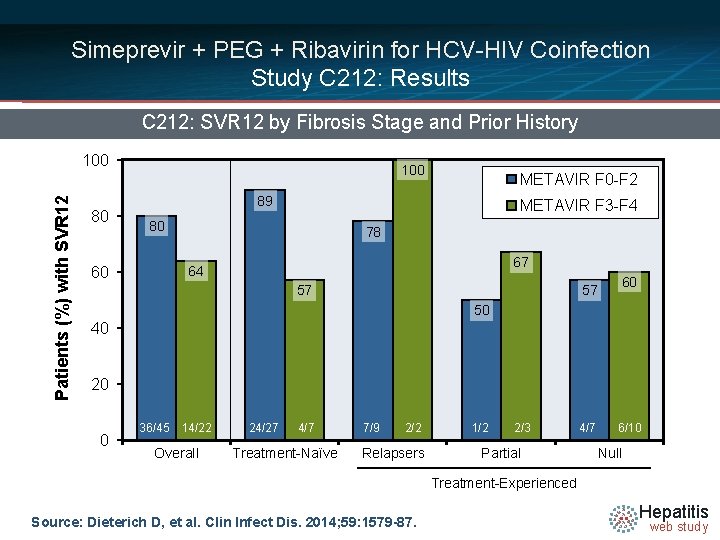
Simeprevir + PEG + Ribavirin for HCV-HIV Coinfection Study C 212: Results C 212: SVR 12 by Fibrosis Stage and Prior History Patients (%) with SVR 12 100 80 100 METAVIR F 0 -F 2 89 METAVIR F 3 -F 4 80 78 67 64 60 57 57 60 50 40 20 0 36/45 14/22 Overall 24/27 4/7 Treatment-Naïve 7/9 2/2 Relapsers 1/2 2/3 Partial 4/7 6/10 Null Treatment-Experienced Source: Dieterich D, et al. Clin Infect Dis. 2014; 59: 1579 -87. Hepatitis web study

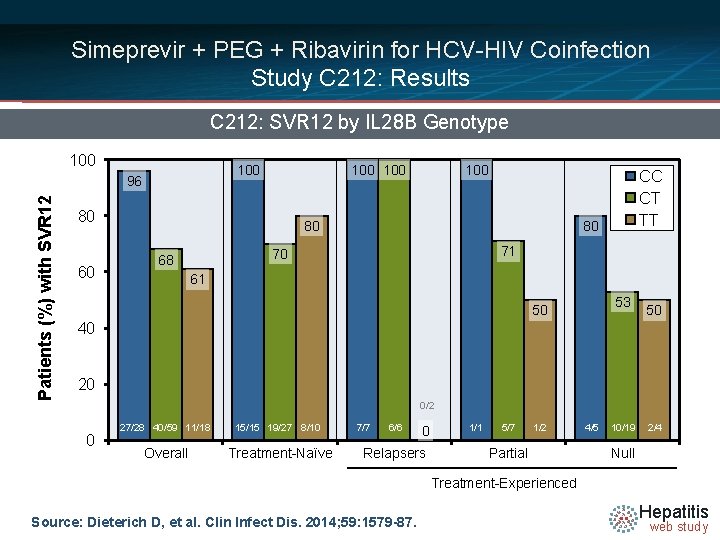
Simeprevir + PEG + Ribavirin for HCV-HIV Coinfection Study C 212: Results C 212: SVR 12 by IL 28 B Genotype 100 Patients (%) with SVR 12 96 100 80 60 100 80 80 71 70 68 CC CT TT 61 53 50 50 40 20 0/2 0 27/28 40/59 11/18 15/15 19/27 8/10 Overall Treatment-Naïve 7/7 6/6 0 Relapsers 1/1 5/7 1/2 Partial 4/5 10/19 2/4 Null Treatment-Experienced Source: Dieterich D, et al. Clin Infect Dis. 2014; 59: 1579 -87. Hepatitis web study

Simeprevir + PEG + Ribavirin for HCV-HIV Coinfection Study C 212: Conclusions: “Simeprevir was generally well tolerated with safety similar to that observed in HCV-monoinfected patients and high SVR 12 rates in HCV treatment-naive patients, prior relapsers, prior partial responders, and prior null responders with HIV-1 coinfection. ” Source: Dieterich D, et al. Clin Infect Dis. 2014; 59: 1579 -87. Hepatitis web study

This slide deck is from the University of Washington’s Hepatitis C Online and Hepatitis Web Study projects. Hepatitis C Online www. hepatitisc. uw. edu Hepatitis Web Study http: //depts. washington. edu/hepstudy/ Funded by a grant from the Centers for Disease Control and Prevention. Hepatitis web study
 Mobile phase and stationary phase
Mobile phase and stationary phase Stationary phase and mobile phase in hplc
Stationary phase and mobile phase in hplc Chromatography mobile phase and stationary phase
Chromatography mobile phase and stationary phase Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Tswett pronunciation
Tswett pronunciation Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Power formula three phase
Power formula three phase In a ∆-connected source feeding a y-connected load
In a ∆-connected source feeding a y-connected load Broad phase vs narrow phase
Broad phase vs narrow phase Intensive phase of tb treatment
Intensive phase of tb treatment

















