PHARMACY LAWS 1 Pharmacy Laws Federal law takes


















- Slides: 19

PHARMACY LAWS 1

Pharmacy Laws Federal law takes precedence over state law unless the state law is stricter than the federal law. The most restrictive law will take precedence.

Pure Food and Drug At 1906 n Enacted in 1906 to prohibit the interstate transportation or sale of adulterated and misbranded food and drugs.

TERMS n n Adulteration: the mishandling of medication that can lead to contamination and cause injury or illness to the consumer. Misbranding: deceptive or misleading labeling of a product that may lead the consumer to believe that the product will cure an illness (illegally labeled).

FOOD, DRUG AND COSMETIC ACT (FDCA 1938) n FDA was created under this act. n Required that all new drugs be filed with FDA. n Clearly defined adulteration and misbranding of drug and food products.

DURHAM-HUMPHREY ACT of 1951 n n Required all products to have adequate directions for use unless they contain the federal legend: “Caution: Federal law prohibits dispensing without prescription. ” It separated drugs into 2 categories: n Legend – requires a prescription n Nonlegend – OTC not requiring a prescription. Allows verbal prescriptions over the telephone. Allows refills to be called in from a physician’s office.

Kefauver-Harris Amendment of 1962 n Requires that all medication on the market in the US be pure, safe and effective.

Comprehensive Drug Abuse Prevention and Control Act of 1970 n The Drug Enforcement Agency (DEA) was created under this act. n Controlled substances were placed into one of five schedules.

Poison Prevention Packaging Act of 1970 n Enacted to reduce the accidental poisoning in children. n Most OTC and legend drugs must be child-resistant.

Occupational Safety and Health Act (OSHA) of 1970 n n n Ensures n Job safety and health standards for employees n Maintain a reporting system for job-related injuries and illness n Reduce hazards in the workplace n Conduct audits to guarantee compliance with act It addressed air contaminants, flammable and combustible liquids, eye and skin protection, and hazard communication standards in the pharmacy. Require usage of the MSDS.

Drug Listing Act of 1972 n n n Created an 11 -digit number to identify each drug. This is the NDC (National Drug Code) number. The first five digits identify the: n Manufacturer The next four digits identify the: n Drug product The final two digits represent the: n Package size and packaging

Orphan Drug Act of 1983 n Orphan drugs are medications for rare diseases (<200, 000 cases in the world). n The law provides tax incentives and exclusive licensing to develop and market orphan medications.

Drug Price Competition and Patent Term Restoration Act of 1984 n Encouraged the creation of both generic and new medication. n Streamlined the process for generic drug approval and extended patent licenses.

Prescription Drug Marketing Act of 1987 n n n Prohibits the reimportation of a drug into the United States by anyone except the manufacturer. Prohibits the sale or distribution of samples to anyone other than those licensed to prescribe them. Requires that all medications for animals are to be labeled with: “Caution: Federal law restricts this drug to use by or on an order of a licensed veterinarian. ”

Anabolic Steroid Control Act of 1990 n Created more strict penalties for the abuse of anabolic steroids and their misuse by athletes.

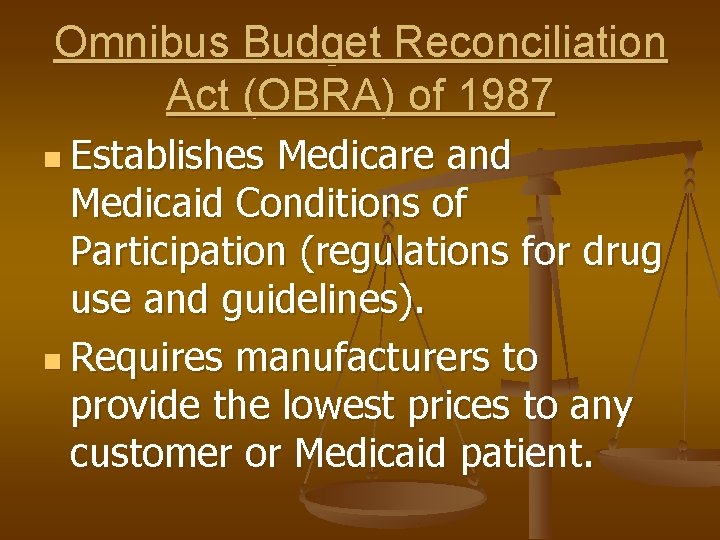
Omnibus Budget Reconciliation Act (OBRA) of 1987 n Establishes Medicare and Medicaid Conditions of Participation (regulations for drug use and guidelines). n Requires manufacturers to provide the lowest prices to any customer or Medicaid patient.

FDA Safe Medical Devices Act of 1990 n Requires that all medical devices be tracked and records be maintained for durable medical equipment, such as infusion pumps.

Americans with Disabilities Act (ADA) of 1990 Prevents discrimination against potential employees who may possess a disability. n The business must make reasonable accommodations for the employee. n

Health Insurance Portability and Accountability Act (HIPAA) There 1. 2. are two parts of HIPAA: Protects health insurance coverage for workers and families when they change or lose their jobs. Administrative Simplification – electronic transactions and required Health Information Privacy.
 Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law
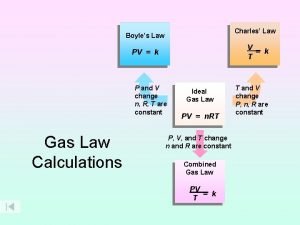
Newton's first law V=k/p
V=k/p Avogadro's law constants
Avogadro's law constants Rear right safety restraint system fault
Rear right safety restraint system fault Useless laws weaken the necessary laws
Useless laws weaken the necessary laws Dea number verification
Dea number verification Application of colligative properties in pharmacy
Application of colligative properties in pharmacy How does newton's law of gravity extend kepler's laws
How does newton's law of gravity extend kepler's laws Mendelian principles of genetics
Mendelian principles of genetics Law 32 48 laws of power
Law 32 48 laws of power An old conveyor belt takes 21
An old conveyor belt takes 21 It is the measure of how much space an object takes up.
It is the measure of how much space an object takes up. Tucker turtle technique
Tucker turtle technique The lamb of god who takes away the sin of the world
The lamb of god who takes away the sin of the world The characters struggle takes place
The characters struggle takes place Flowchart of respiratory system
Flowchart of respiratory system What is the setting of ratatouille
What is the setting of ratatouille Eclectic approach psychology
Eclectic approach psychology Green plants make their own food by photosynthesis
Green plants make their own food by photosynthesis