Personalized Medicine in the ICU Asim Siddiqui Sirius





- Slides: 14

Personalized Medicine in the ICU Asim Siddiqui Sirius Genomics 13 th September 2007 VANBUG

Developing and commercializing rapid, DNA-based diagnostic (Dx) and pharmacogenetic (PGx) tests that will revolutionize critical care medicine.

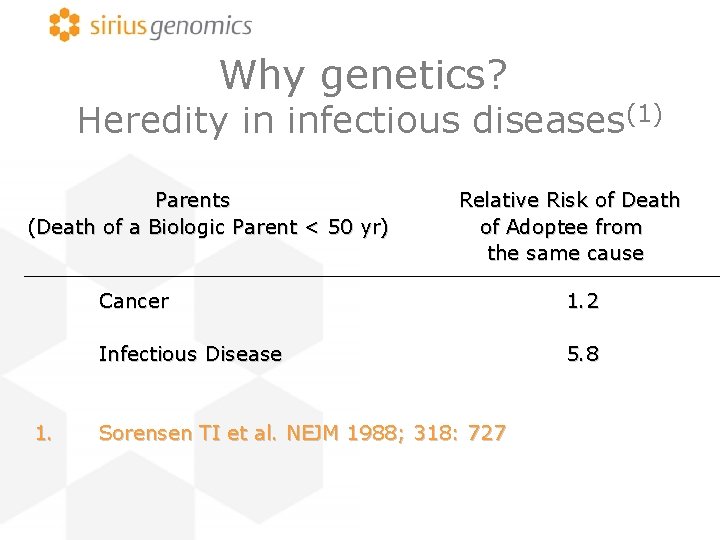
Why genetics? Heredity in infectious diseases(1) Parents (Death of a Biologic Parent < 50 yr) 1. Relative Risk of Death of Adoptee from the same cause Cancer 1. 2 Infectious Disease 5. 8 Sorensen TI et al. NEJM 1988; 318: 727

APC (Activated Protein C) Xigris® § § § Severe sepsis, high risk of death Uptake: 5% of target population Concern re: efficacy Concern re: safety Physicians have difficulty determining who gets the drug

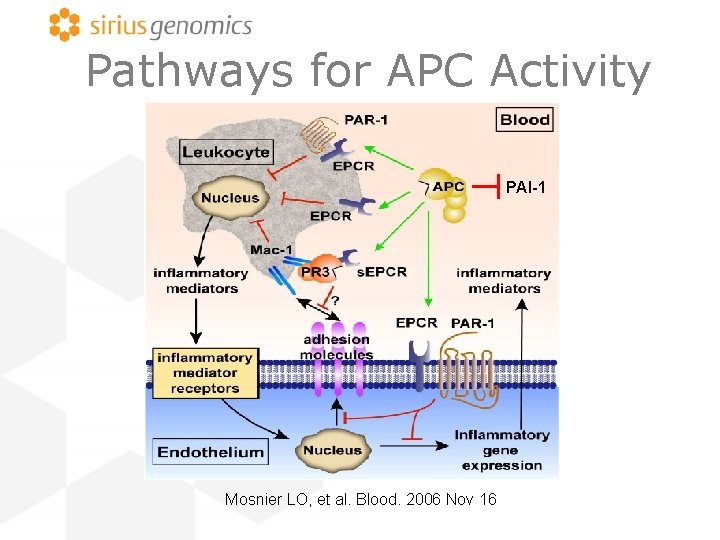
Pathways for APC Activity PAI-1 Mosnier LO, et al. Blood. 2006 Nov 16

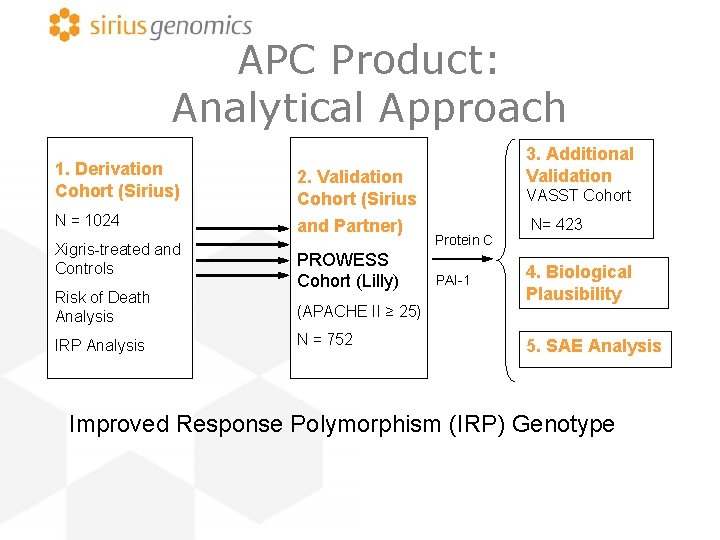
APC Product: Analytical Approach 1. Derivation Cohort (Sirius) 2. Validation Cohort (Sirius N = 1024 and Partner) Xigris-treated and Controls Risk of Death Analysis IRP Analysis PROWESS Cohort (Lilly) (APACHE II ≥ 25) N = 752 3. Additional Validation VASST Cohort Protein C PAI-1 N= 423 4. Biological Plausibility 5. SAE Analysis Improved Response Polymorphism (IRP) Genotype

IRP Definition § rs 2069912 ‘C’ allele efficacious response § rs 7242 ‘T’ allele efficacious response § 1 or more copy of each ‘+/+’ § 1 or more copy of only one ‘+/-’ § Zero copies of each ‘-/-’

Absolute Risk Reduction (ARR) Across Three Cohorts Improved Response Polymorphism (IRP) Genotype

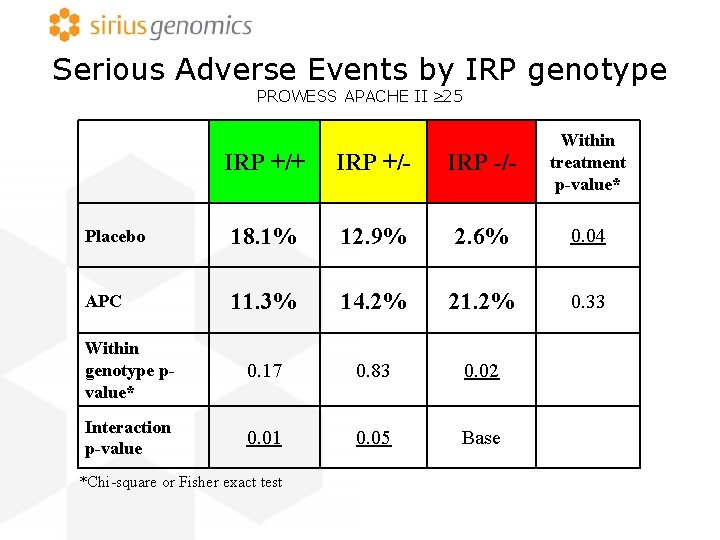
Serious Adverse Events by IRP genotype PROWESS APACHE II 25 IRP +/+ IRP +/- IRP -/- Within treatment p-value* Placebo 18. 1% 12. 9% 2. 6% 0. 04 APC 11. 3% 14. 2% 21. 2% 0. 33 Within genotype pvalue* 0. 17 0. 83 0. 02 Interaction p-value 0. 01 0. 05 Base *Chi-square or Fisher exact test

Efficacy, Biology, SAEs +/+ +/- -/- +/+ +/- -/- Improved Response Polymorphism (IRP) Genotype +/+ +/- -/-

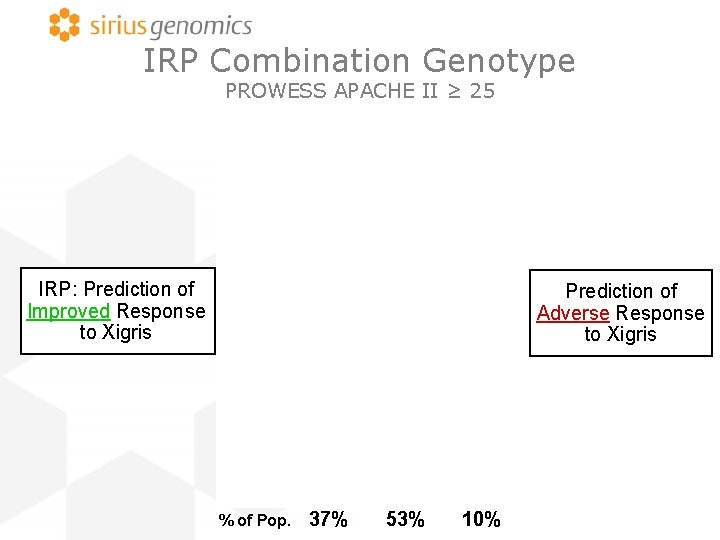
IRP Combination Genotype PROWESS APACHE II ≥ 25 IRP: Prediction of Improved Response to Xigris Prediction of Adverse Response to Xigris % of Pop. 37% 53% 10%

That’s where the science ends but….

Platform & Regulatory Process § Identify a suitable platform – 45 mins from blood sample to genotype – Fully automated – CLIA-waived – Hospital lab or point-of-care § FDA approval for test § Further studies

Acknowledgements § § § § Jim Russell Keith Walley Tony Gordon Karen Mooder Hugh Wellman Marissa Le. Blanc Xuekui Zhang Bill Macias Mark Williamson Sandra Kirkwood Nicholas Lewin. Koh § Lee O’Brian § §



























