Patent settlements reach the US Supreme Court The





















- Slides: 28

Patent settlements reach the US Supreme Court The US Supreme Court in FTC v. Actavis (June 17, 2013) - Context, facts, outcome & implications - Patent reform - Where, When, Why and How? Associate Prof. Dr. LL. M. Timo Minssen Centre for Information and Innovation Law University of Copenhagen June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 1

Introduction • After EU Commission’s pharma sector report (2009) competition law increasingly important to pharma sector. • One of the most debated issues: the practice of patent settlements (a. k. a. “pay for delay” or “reverse payments”). • cf. 3 rd Commission report on patents settlements (July 25 th, 2012) & pending Lundbeck decision (focus of CIIR update presentation in Nov. 2012). • Also important US developments on this issue • Focus of this presentation: The US Supreme Court decision in FTC v. Actavis June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 2

AGENDA 1) Patent settlements: What is at stake? 2) Recent US developments: Legal framework, policy statements and the split at the CAFC 3) US Supreme Court in FTC v. Actavis • • • Procedural history Outcome Practical implications & reactions 4) Conclusions (& comparison to Europe) 5) Comments & Discussion June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 3

Definitions: “Pay for delay”settlements (a. k. a. “patent settlements” or “reverse payments”) • settlements between brand-name drug manufacturers & generic rivals. • resolving patent infringement suits by brand-name drug manufacturer against generic drug-manufacturers for the latter’s attempt to market a competing generic. • involve — like all settlements — risk assessment and business judgment: brand-name drug manufacturers make calculated decisions not to take a chance that the generic firm might convince courts that relevant patent is invalid or not infringed by generic. • Instead, brand-name producer pays or bestows some other benefit upon the generic firm to convince it to abandon its challenge and, in some cases, to delay entry. . June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 4

Main arguments: Pro/Contra- II Pro (Big Pharma & generic industry) • A patent/pay-for-delay settlement may avoid the cost of litigation and creates certainty that allows parties to plan and invest into new products. Both the settling parties and, importantly, society in general may benefit (also acknowledged by 3 rd EU Commission report and US Supreme Court). Contra (EU Commission & FTC) • With such agreements, an originator company removes generic companies' incentives to compete - or to challenge the patent - by transferring money to generic producer. • Elimination or delay of cheaper generic competition through significant payments or other benefits to a generic company can lead to substantial consumer harm and may keep bad patents artificially alive. • Such agreements reduces competitive pressure exercised by (potential) generic market entry in exchange of sharing the originator company's monopoly rents. It replaces competition by collusion, and is thus a restriction of competition. June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 5

US June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 6

The Drug Price Competition and Patent Term Restoration Act of 1984 (Hatch. Waxman Act or Act) • creates special procedures for identifying and resolving patent disputes between brand-name and generic drug manufacturers. • one of these procedure requires a prospective generic manufacturer to assure the Food and Drug Administration (FDA) that it will not infringe the brand-name’s patents. • One way to provide such assurance [under 21 U. S. C. § 355(j)(2)(A)(vii)(IV), i. e. the “paragraph IV” route] is - by certifying that any listed, relevant patent “is invalid, or - will not be infringed by the manufacture, use, or sale” of the generic drug. • Provides additional incentives for generic producer to sue ”bad patents” • Rationale of Hatch Waxman in conflict with patent settlements? June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 7

Summary of recent developments • Ban on such agreements being part Obama administration's budget. FTC follows this policy. • BUT: Until recently neither Congress nor courts adopted FTC's stance on the purported anti-competitiveness of such agreements. • Most courts adopted “scope of patent”- test (FTC v. Watson – now Actavis) and held that on average, generic drugs actually come on the market sooner than they would if the patentee retained its exclusivity for full scope of the patent term. • If found anticompetive… mostly very unusual behaviour of parties • However, In re K-Dur, 686 F. 3 d 197 (3 d Cir. July 16, 2012) basically followed FTC approach with a “quick look”- test and a presumption of invalidity. • FTC v. Actavis and K-Dur signifed split at Federal Circuit. Both cases where appealed to the US Supreme Court (petition for certiorari) • Supreme Court granted certiorari in FTC v. Actavis and rendered decision on June 17, 2013. Created a new third approach: The “rule of reason” -test. June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 8

”Scope of the patent” test and previous treatment of pay for delay • Prior to the Third Circuit’s decision in K-Dur, the weight of appellate authority — and all of the more recent appellate authority — has adopted one form or another of the “scope of the patent test. ” • Under that test, reverse payments are permitted as long as (1) the exclusion does not exceed the patent’s scope, (2) the patent holder’s claim of infringement was not objectively baseless, and (3) the patent was not procured by fraud on the U. S. Patent and Trademark Office (PTO). • Courts of Appeals for 2 nd, 11 th (FTC v. Actavis), and Federal Circuits have adopted this test, rejecting challenges to pay-for-delay settlements where the restrictions in the settlement agreement fell within the scope of the patent with respect to duration and the products covered. • See, e. g. , Ark. Carpenters H. & Welfare Fund v. Bayer AG, 604 F. 3 d 98, 106 (2 d Cir. 2010) (Cipro); In re Ciprofloxacin Hydrochlo-ride Antitrust Litig. , 544 F. 3 d 1323, 1335 -36 (Fed. Cir. 2008); In re Tamoxifen Citrate Antitrust Litig. , 466 F. 3 d 187, 213 -15 (2 d Cir. 2006); Schering-Plough Corp. v. FTC, 402 F. 3 d 1056, 1066 (11 th Cir. 2005). In fact, the Eleventh Circuit’s decision in Schering. Plough arose out of the same set of facts as the Third Circuit’s decision in K-Dur. June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 9

A quick look at the new approach in In re KDur, 686 F. 3 d 197 (3 d Cir. July 16, 2012) The "Quick Look Rule of Reason" Test The "quick look rule of reason" test starts from an opposite perspective, and holds that a reverse payment agreement is "prima facie evidence of an unreasonable restraint of trade. " According to the Third Circuit(writing in the K-Dur case), this presumption could be overcome under one of two circumstances: 1. 2. ! There was no reverse payment because any payment was "for something other than delay of market entry. " The reverse payment offered a competitive benefit, i. e. , it somehow increased competition. As the Third Circuit noted, it will be the "rare" agreement that survives scrutiny under this test! June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 10

FTC V. Actavis – procedural history I • Solvay Pharmaceuticals obtained patent and MA for Andro. Gel (exp. 2020). • Watson (now Actavis) and another generic producer filed applications for generic drugs modeled after Andro. Gel & certified under para IV that Solvay’s patent invalid and not infringed by their drugs. • Solvay sued i. a. Actavis under 35 U. S. C. § 271(e)(2)(A), claiming patent infringement. • FDA eventually approved Actavis’ generic product. • Instead marketing its drug, Actavis entered into a “reverse payment” settlement with Solvay 2006, agreeing not to market its generic for number of years and to promote Andro. Gel in exchange for millions of dollars. • Other generic manufacturers aligned in the patent litigation made a similar agreement with Solvay. June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 11

FTC V. Actavis – procedural history II • FTC filed suit • Feb. 2010: • April 2012: Eleventh Circuit concluded that as long as the anticompetitive effects of a settlement fall within the scope of the patent’s exclusionary potential, the settlement is immune from antitrust attack. • Court affirmed the complaint’s dismissal noting that the FTC had neither alleged nor shown that challenged agreement excluded competition to greater extent than would the patent, if valid. • Recognized that if no settlement, a court might hold patent invalid. But since public policy favors settlement of disputes, courts could not require parties to continue litigating in order to avoid antitrust liability. June 18, 2013 District Court dismissed complaint. Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 12

Questions referred to the Supreme Court • The FTC sought certiorari • Question: Whether reverse-payment agreements are per se lawful unless the underlying patent litigation was a sham or the patent was obtained by fraud (as the court below held), or instead are presumptively anticompetitive and unlawful (as the Third Circuit has held). • US Supreme Court granted the FTC’s petition, since different courts have reached different conclusions about the application of the antitrust laws to Hatch-Waxman-related patent settlements, . Compare, e. g. , id. , at 1312 (case below) (settlements generally “immunefrom antitrust attack”); In re Ciprofloxacin Hydrochloride Antitrust Litigation , 544 F. 3 d 1323, 1332– 1337 (CA Fed. 2008) (similar); In re Tamoxifen Citrate Antitrust Litigation, 466 F. 3 d 187, 212– 213 (CA 2 2006) (similar), with In re K-Dur Antitrust Litigation, 686 F. 3 d 197, 214– 218 (CA 3 2012) (settlements presumptively unlawful). • Decision was rendered yesterday (17 June 2013) June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 13

Abstract of the outcome • 5 -3 opinion delivered by Justice Breyer (joined in by Justices Kennedy, Ginsburg, Sotomayor, and Kagan) • In a three-part holding the Court declined to hold that reverse payment settlement agreements are presumptively unlawful, and that “Courts should apply a “rule of reason”, rather than a “quick look” approach. ” • Yet, the Court also held that the FTC should have been allowed to prove its antitrust claims and that the exclusionary potential of a patent does not immunize drug patent settlement agreements from antitrust attack. • The FTC almost immediately issued a press release hailing the decision as a “significant victory. ” June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 14

FTC v. Actavis: The three-part holding by the Supreme Court (5 -3) I A) Although the anticompetitive effects of the reverse settlement agreement might fall within the scope of the exclusionary potential of Solvay’s patent, this does not immunize the agreement from antitrust attack. June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 15

FTC v. Actavis: The three-part holding by the Supreme Court (5 -3) II B) 5 considerations lead to conclusion that the (partially acknowledged) concerns by the 11 th Circuit should not determine the result here and that FTC should have been given the opportunity to prove its antitrust claim. 1. the specific restraint at issue has the “potential for genuine adverse effects on competition”. 2. these anticompetitive consequences will at least sometimes prove unjustified. 3. where a reverse payment threatens to work unjustified anticompetitive harm, the patentee likely has the power to bring about that harm in practice. The size of the payment from a branded drug manufacturer to a generic challenger is a strong indicator of such power. 4. an antitrust action might be more feasible administratively than the Eleventh Circuit believed. 5. the fact that a large, unjustified reverse payment risks antitrust liability does not prevent litigating parties from settling their lawsuits. June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 16

FTC v. Actavis: The three-part holding by the Supreme Court (5 -3) III C) Court declines to hold that reverse payment settlement agreements are presumptively unlawful. Courts reviewing such agreements should proceed by applying the “rule of reason”, rather than under a “quick look” approach. See California Dental Assn. v. FTC, 526 U. S. 756, 775, n. 12. Pp. 20 -21 June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 17

Dissent by C. J. Roberts (joined by J. Scalia & JThomas (J. Alito recused) • “settling a patent claim cannot possibly impose unlawful anticompetitive harm if the patent holder is acting within the scope of a valid patent and therefore permitted to do precisely what the antitrust suit claims is unlawful“ • "Good luck to the district courts that must, when faced with a patent settlement, weigh the 'likely anticompetitive effects, redeeming virtues, market power, and potentially offsetting legal considerations present in the circumstances. '“ • ” decision may very well discourage generics from challenging pharmaceutical patents in the first place” June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 18

FTC v. Actavis- Implications & reactions • More legal uncertainty/ambiguity in the name of utilitarian goals • As pointed out in the dissent: district-courts face a tough job • In turn: brand generic companies will have to determine on a case-bycase basis whether and how to structure drug patent settlement arrangements. Any agreement might be reviewed……. • Analyst Ronny Gal (Sanford Bernstein): "If I were a patent attorney in the drug world, I would be opening a bottle of Champagne right now. It's basically a 'full-employment of patent attorneys' decision. " • The five factors identified by the court require particular caution June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 19

Reactions FDA: ”The Supreme Court’s decision is a significant victory for American consumers, American taxpayers, and free markets. The Court has made it clear that pay-for-delay agreements between brand generic drug companies are subject to antitrust scrutiny, …” Ph. RMA: Glad that FTC’s “presumption of invalidity” attempt was unanimously rejected, BUT the “Court’s decision creates a degree of uncertainty that will make it less likely that innovator pharmaceutical and generic companies will be able to settle these disputes in the future. ” GPh. A: “the Court’s ruling will require generic companies to take on a greater administrative burden to pursue a patent challenge, potentially lowering the number of challenges. As a result, consumers may have access to fewer generic options. ” Actavis: “althought it “does place an additional and unnecessary administrative burden on our industry”, it “continues to provide for a lawful and legitimate pathway for resolving patent challenge litigation in a manner that is procompetitive and beneficial to American consumers. ” June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 20

Conclusions, Comments & Discussion • The Supreme Court refused to give the FTC what it wanted: a complete ban on patent settlements. But also gave the agency the right to continue pursuing legal challenges to individual settlements. • Majority failed to provide clear and unambiguous guidance as to how patent settlements could be structured to avoid antitrust exposure short of litigating a patent dispute to the end. • In practical terms, we will see a lot of continued litigation as FTC will continue to challenge these agreements • More class-action lawsuits filed to challenge patent settlements because of the Supreme Court ruling. • Does the evolving EU approach resemble the US ”rule of reason” approach? (3 rd PS report, Commisson’s objections and pending Lundbeck decision). June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 21

June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 22

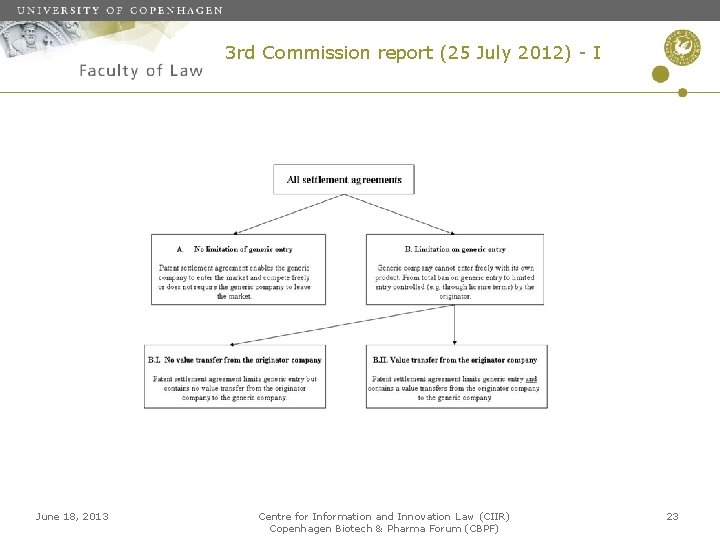
3 rd Commission report (25 July 2012) - I June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 23

June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 24

June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 25

Alternatives to antitrust/competition law based control of patent settlements/reverse payments ? ? ? ? Gregory Dolin, REVERSE SETTLEMENTS AS PATENT INVALIDITY SIGNALS, Harvard Journal of Law & Technology Volume 24 (Number 2 Spring 2011) June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 26

Any questions or comments? Thank you for your attention ! E-mail: Web: Timo. Minssen@jur. ku. dk www. ciir. dk CPH Summer School in Pharma Law & Policy (Aug. 12 -16, 2013): http: //copenhagensummeruniversity. ku. dk/en/courses/pharmalawpolicy/ KU course in EU Pharma Law, IPR & the Life Sciences: https: //sis. ku. dk/kurser/viskursus. aspx? knr=139161 June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 27

June 18, 2013 Centre for Information and Innovation Law (CIIR) Copenhagen Biotech & Pharma Forum (CBPF) 28
 How cases reach the supreme court worksheet answers
How cases reach the supreme court worksheet answers Is there a basketball court above the supreme court
Is there a basketball court above the supreme court Supreme court justice system
Supreme court justice system John marshall supreme court
John marshall supreme court Supreme court does
Supreme court does Supreme court cases graphic organizer answers
Supreme court cases graphic organizer answers Supreme court
Supreme court The supreme court change
The supreme court change Apush amendments review
Apush amendments review Supreme court does
Supreme court does What do these headlines say about how the supreme court
What do these headlines say about how the supreme court Which of these best summarizes the monroe doctrine?
Which of these best summarizes the monroe doctrine? Supreme court
Supreme court Hierarchy of criminal courts in india
Hierarchy of criminal courts in india Supreme court cases apush
Supreme court cases apush Have supreme court
Have supreme court Vocabulary activity 12 supreme court decision making
Vocabulary activity 12 supreme court decision making John marshall
John marshall Insular cases apush
Insular cases apush The u.s. supreme court works chiefly as a(n)
The u.s. supreme court works chiefly as a(n) The supreme court
The supreme court Geography grade 12 settlement
Geography grade 12 settlement East india company flag
East india company flag Spanish settlements in north america
Spanish settlements in north america English settlements in america
English settlements in america Citystate informal settlements
Citystate informal settlements Linear settlement pattern
Linear settlement pattern Danish settlements in india
Danish settlements in india Where are the vikings from
Where are the vikings from