Paracoccus denitrificans Drew Meyers Agriculture 73 17 from








- Slides: 16

Paracoccus denitrificans Drew Meyers


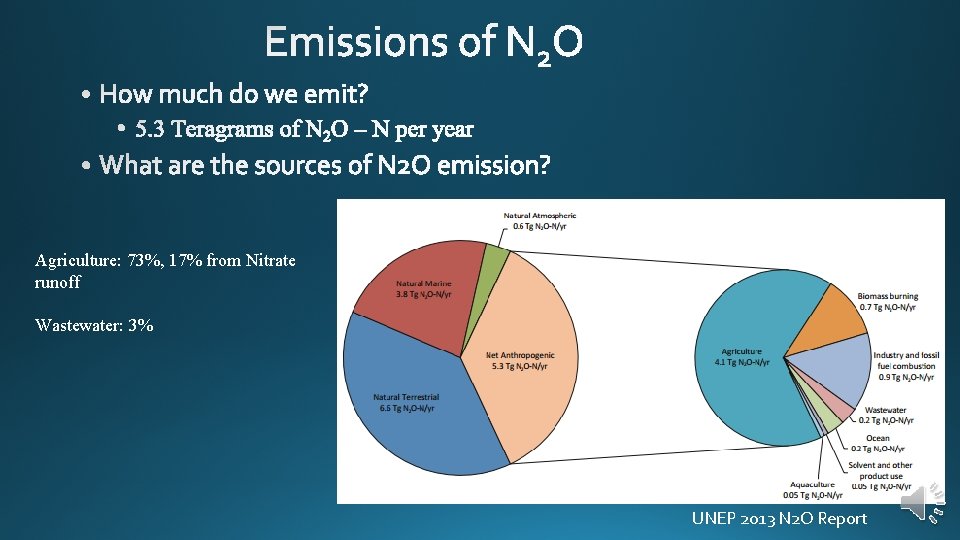
Agriculture: 73%, 17% from Nitrate runoff Wastewater: 3% UNEP 2013 N 2 O Report



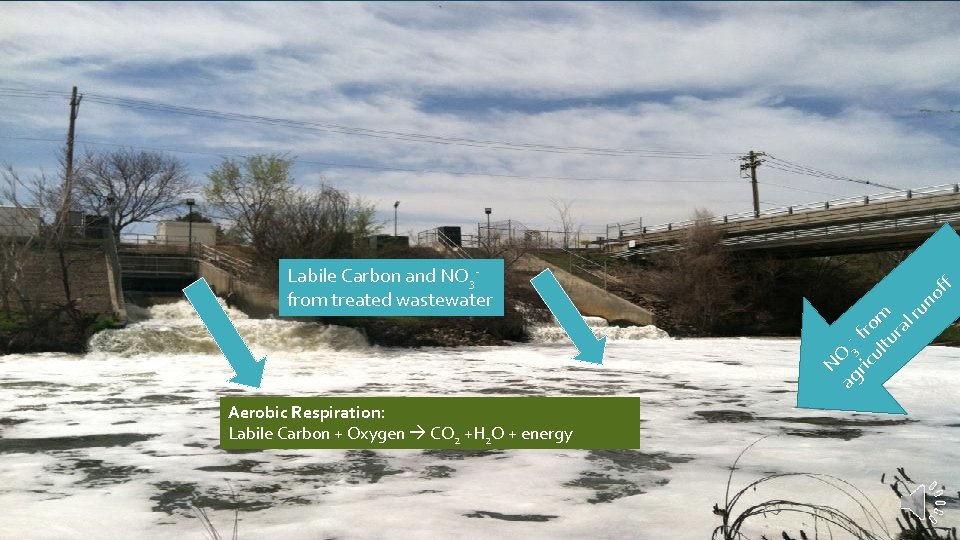
Labile Carbon and NO 3 from treated wastewater

f of ag 3 ric fro ul m tu ra lr un NO Labile Carbon and NO 3 from treated wastewater

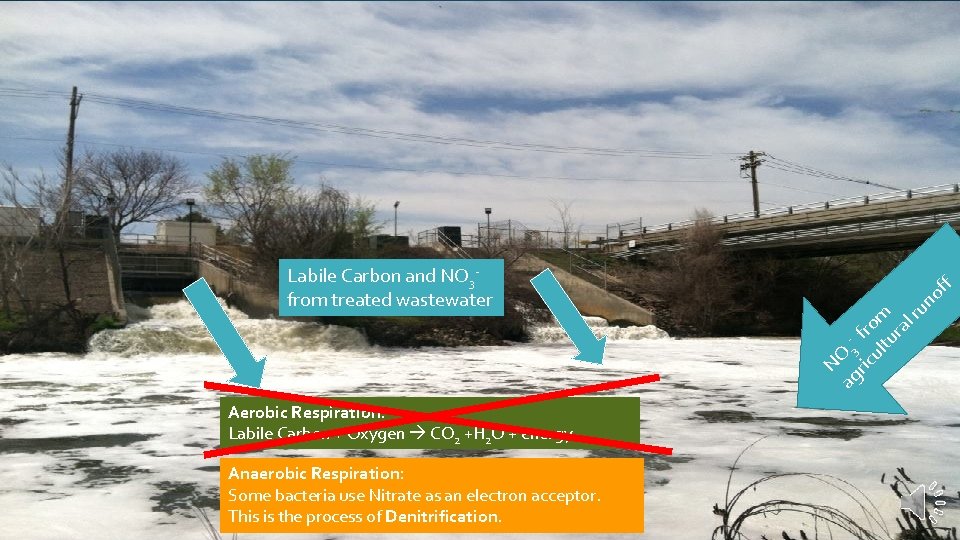
f of ag 3 ric fro ul m tu ra lr un NO Labile Carbon and NO 3 from treated wastewater Aerobic Respiration: Labile Carbon + Oxygen CO 2 +H 2 O + energy

f of ag 3 ric fro ul m tu ra lr un NO Labile Carbon and NO 3 from treated wastewater Aerobic Respiration: Labile Carbon + Oxygen CO 2 +H 2 O + energy Anaerobic Respiration: Some bacteria use Nitrate as an electron acceptor. This is the process of Denitrification.

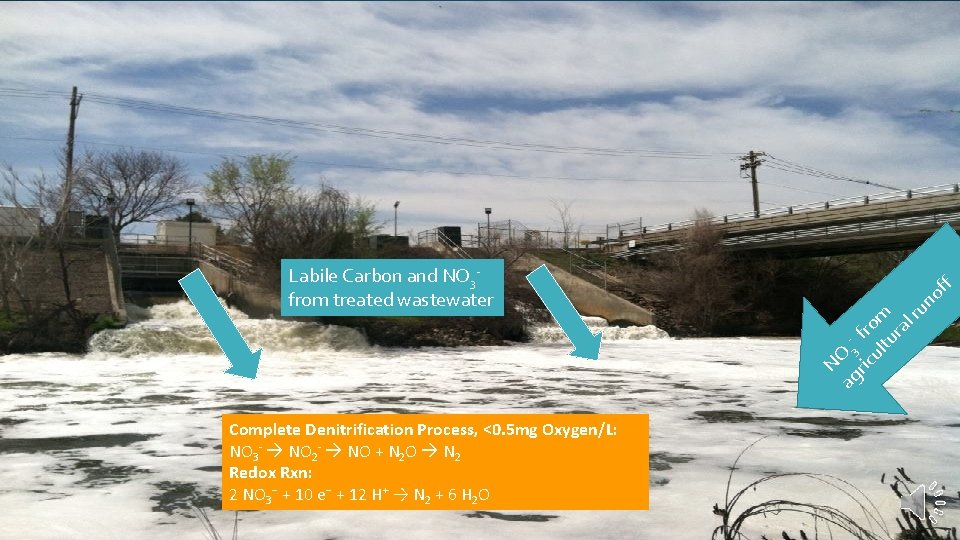
f of ag 3 ric fro ul m tu ra lr un NO Labile Carbon and NO 3 from treated wastewater Complete Denitrification Process, <0. 5 mg Oxygen/L: NO 3 - NO 2 - NO + N 2 O N 2 Redox Rxn: 2 NO 3− + 10 e− + 12 H+ → N 2 + 6 H 2 O

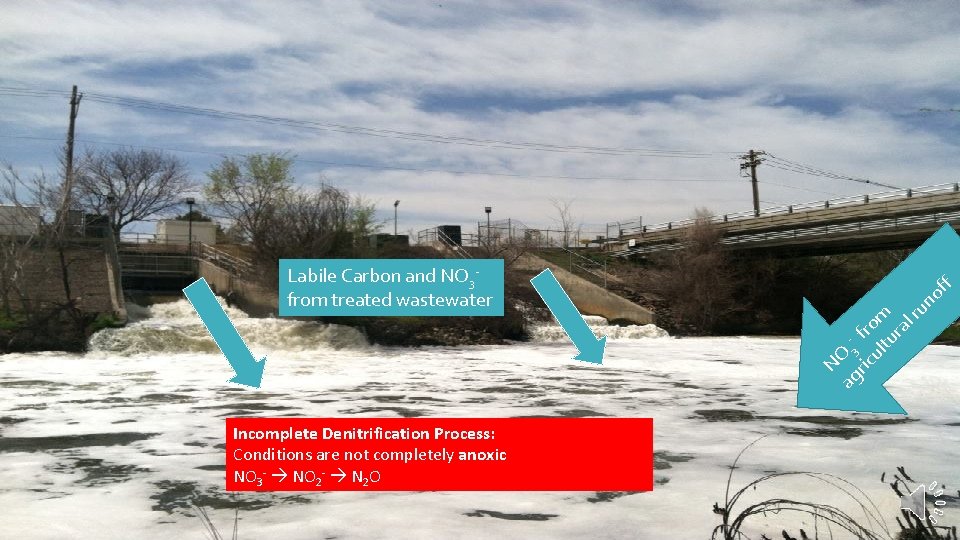
f of ag 3 ric fro ul m tu ra lr un NO Labile Carbon and NO 3 from treated wastewater Incomplete Denitrification Process: Conditions are not completely anoxic NO 3 - NO 2 - N 2 O

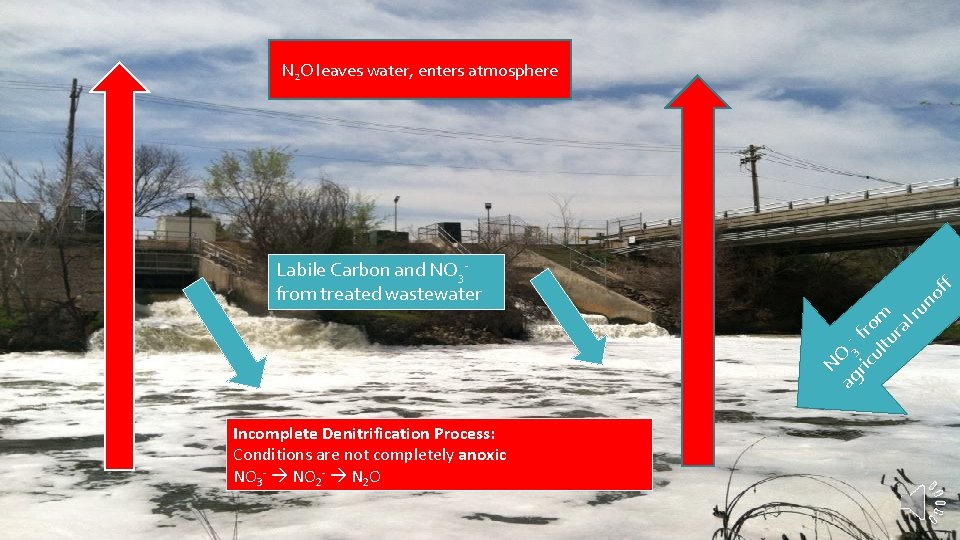
of ag 3 ric fro ul m tu ra lr un NO Labile Carbon and NO 3 from treated wastewater f N 2 O leaves water, enters atmosphere Incomplete Denitrification Process: Conditions are not completely anoxic NO 3 - NO 2 - N 2 O

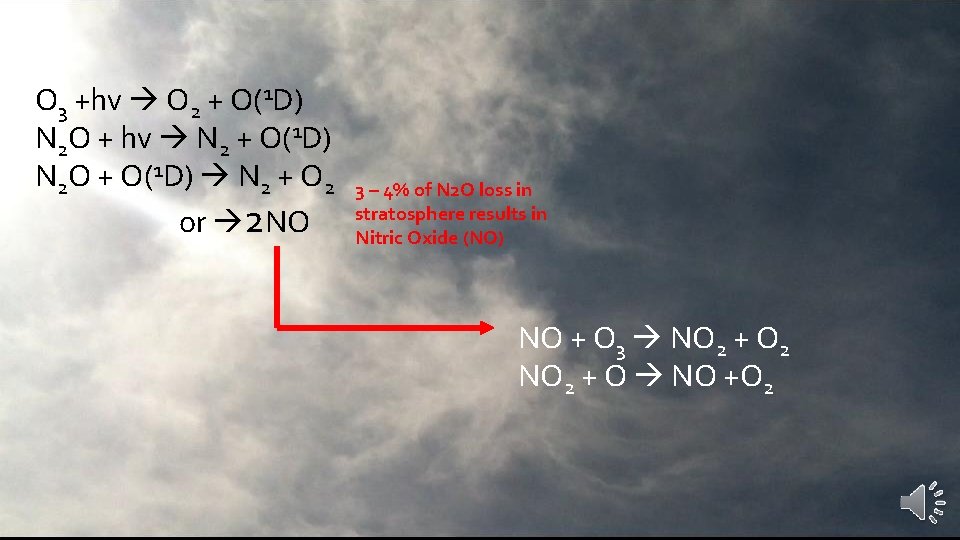
O 3 +hv O 2 + O(1 D) N 2 O + hv N 2 + O(1 D) N 2 O + O(1 D) N 2 + O 2 or 2 NO 3 – 4% of N 2 O loss in stratosphere results in Nitric Oxide (NO)

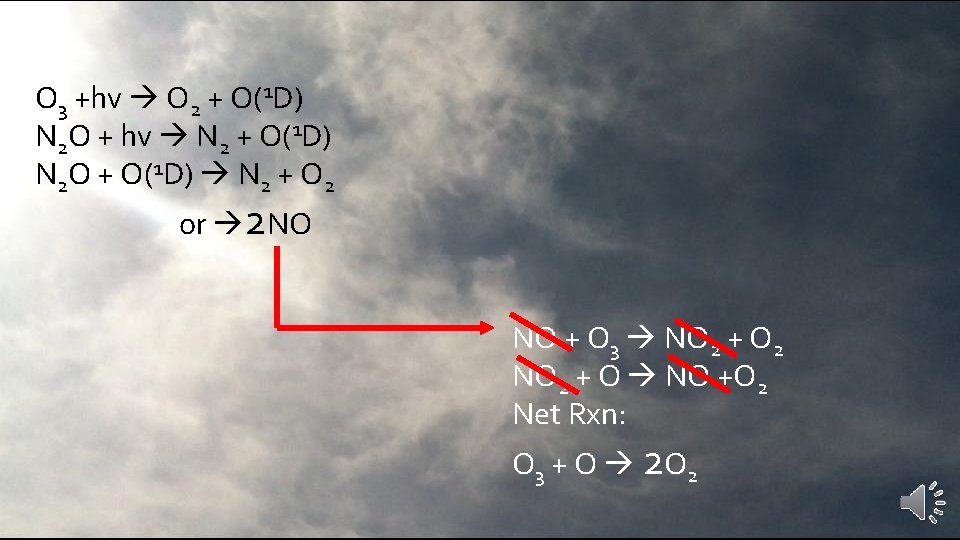
O 3 +hv O 2 + O(1 D) N 2 O + hv N 2 + O(1 D) N 2 O + O(1 D) N 2 + O 2 or 2 NO 3 – 4% of N 2 O loss in stratosphere results in Nitric Oxide (NO) NO + O 3 NO 2 + O 2 NO 2 + O NO +O 2

O 3 +hv O 2 + O(1 D) N 2 O + hv N 2 + O(1 D) N 2 O + O(1 D) N 2 + O 2 or 2 NO NO + O 3 NO 2 + O 2 NO 2 + O NO +O 2 Net Rxn: O 3 + O 2 O 2