Overview of use of routinely collected health data
















- Slides: 28

Overview of use of routinely collected health data in trials and an introduction to the 3 topic areas covered in the workshop Ian Ford Director, Robertson Centre for Biostatistics and Glasgow Clinical Trials Unit

The old ways?

The old ways?

Newer ways of working

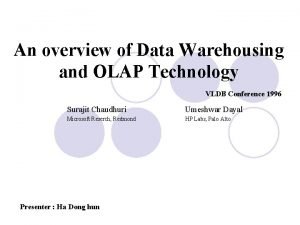
Newer ways of working Sponsor Appoint committees Pharmacovigilance Site setup & administration Monitoring Views data and study reports Trial management Trial Site Research Nurse Enter s dat Rand a omis ation Study co-ordinator IDMC ion nat i sem Dis ata of d Endpoints Committee on WEB portal ti dica adju ata Views d Glasgow Clinical Trials Unit Study Database Web portal development & maintenance Data Management Statistical analysis

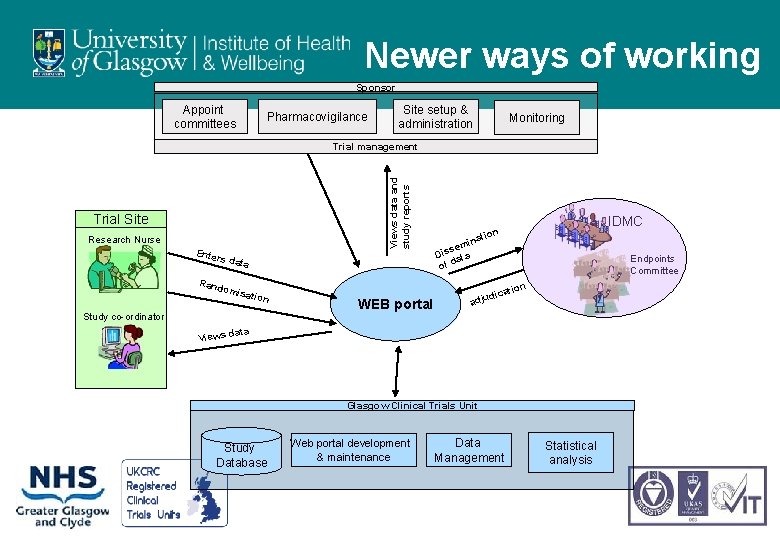
Opportunity • • • • Mothers ante-natal records Maternity Neonatal record Register birth - NHS number Register with GP - CHI GP Appointments Outpatients A&E attendance General hospital admission Prescribing Cancer registration Cancer treatment Community care Death

Scope of the workshop • Using routinely collected health records in support of trials – Assessing feasibility/ supporting design – Facilitating recruitment – Supporting follow-up and data acquisition – within and post trial

Assessing feasibility/ supporting design • Identifying availability of patients • Estimating event rates • Routinely collected data almost always over estimates the availability of patients and the number of events they will have • Can we develop better algorithms?

Facilitating recruitment • Identification of potentially recruits based on key inclusion/ exclusion criteria • Requires a moderately rich phenotype • Or, focus on one or two key variables • Can we create a UK-wide sampling frame from medical history, lifestyle, demographics, lab and prescribing data? – Ideally linking primary and secondary care data.

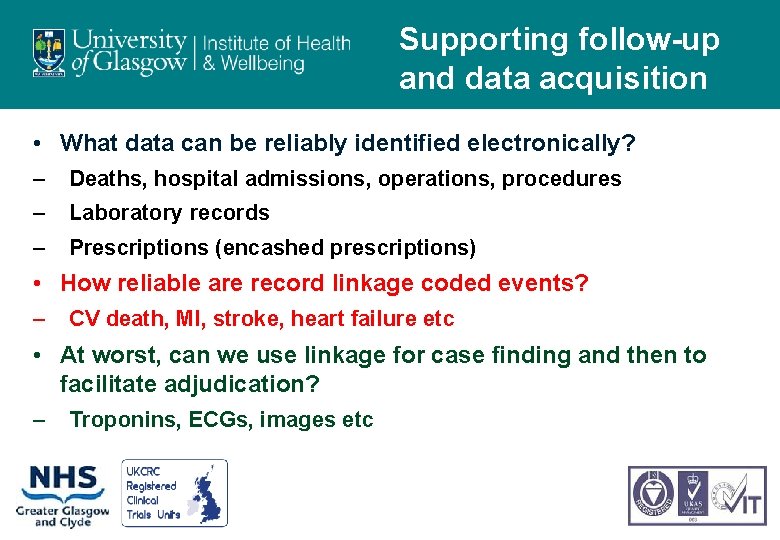
Supporting follow-up and data acquisition • What data can be reliably identified electronically? – Deaths, hospital admissions, operations, procedures – Laboratory records – Prescriptions (encashed prescriptions) • How reliable are record linkage coded events? – CV death, MI, stroke, heart failure etc • At worst, can we use linkage for case finding and then to facilitate adjudication? – Troponins, ECGs, images etc

Extended follow-up • Extended follow-up post trial to assess long-term safety, benefit and cost effectiveness • Deaths, hospital admissions, operations and procedures, incident cancers, prescribing, care homes

• Trials – WOSCOPS and PROSPER – landmark lipid lowering trials – SCOT and FAST – post-approval safety trials – HOOPS – pharmacy based intervention to enhance treatment of heart failure – REMOVAL and TRUST – academic international trials in Type I diabetics and borderline hypothyroid individuals

• Assessing feasibility – TRUST – laboratory records (SCI Store) searched to indentify patients ≥ 65 years of age with ‘recent’ TSH levels in range 4. 5 -19. 9 m. U/L – REMOVAL – Scotland has a national diabetes registry that can be searched to identify potentially eligible patients Type I diabetes who have been given consent to be contacted about trials

• Facilitating recruitment – TRUST – – laboratory records (SCI Store) searched to indentify patients ≥ 65 years of age with ‘recent’ TSH levels in range 4. 5 -19. 9 m. U/L REMOVAL – – national diabetes registry searched to identify Tpye I diabetics in age range SCOT – – GP records searched to identify patients without a history of stroke or CHD, ≥ 65 years of age with history of chronic NSAID use FAST – GP records searched to identify patients ≥ 60 years of age, taking allopurinol (as a surrogate for diagnosis of gout)

• Supporting in-trial follow-up – HOOPS (pharmacy led cluster trial to optimise HF therapy) – – Prescribing records extracted from GP systems Hospitalisations and deaths extracted from national databases for hospital discharge summaries and deaths – SCOT and FAST – – Hospital discharges summaries and deaths extracted from national registries Used to support SAE reporting and for case finding for study endpoints

Supporting long-term follow-up

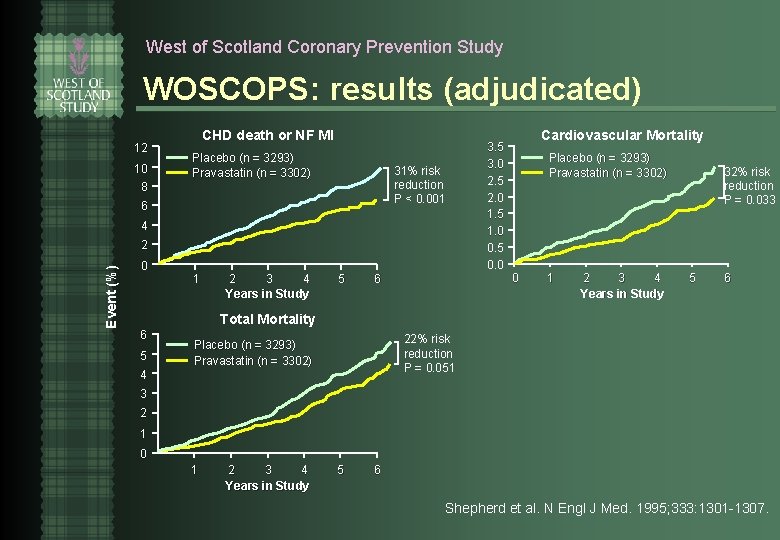
West of Scotland Coronary Prevention Study WOSCOPS: results (adjudicated) 12 10 CHD death or NF MI Placebo (n = 3293) Pravastatin (n = 3302) 31% risk reduction P < 0. 001 8 6 4 Event (%) 2 0 1 2 3 4 Years in Study 5 6 3. 5 3. 0 2. 5 2. 0 1. 5 1. 0 0. 5 0. 0 Cardiovascular Mortality Placebo (n = 3293) Pravastatin (n = 3302) 0 1 2 3 4 Years in Study 32% risk reduction P = 0. 033 5 6 Total Mortality 6 5 4 22% risk reduction P = 0. 051 Placebo (n = 3293) Pravastatin (n = 3302) 3 2 1 0 1 2 3 4 Years in Study 5 6 Shepherd et al. N Engl J Med. 1995; 333: 1301 -1307.

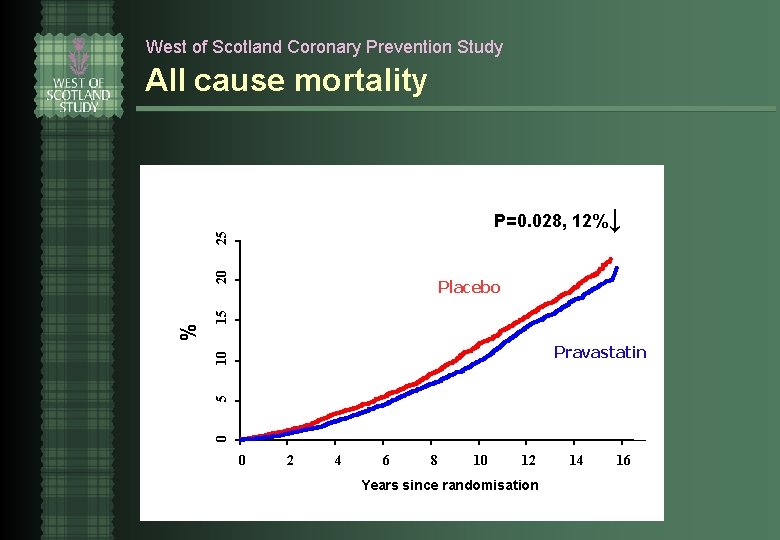
West of Scotland Coronary Prevention Study All cause mortality 15 Placebo 5 10 Pravastatin 0 % 20 25 P=0. 028, 12%↓ 0 2 4 6 8 10 12 Years since randomisation 14 16

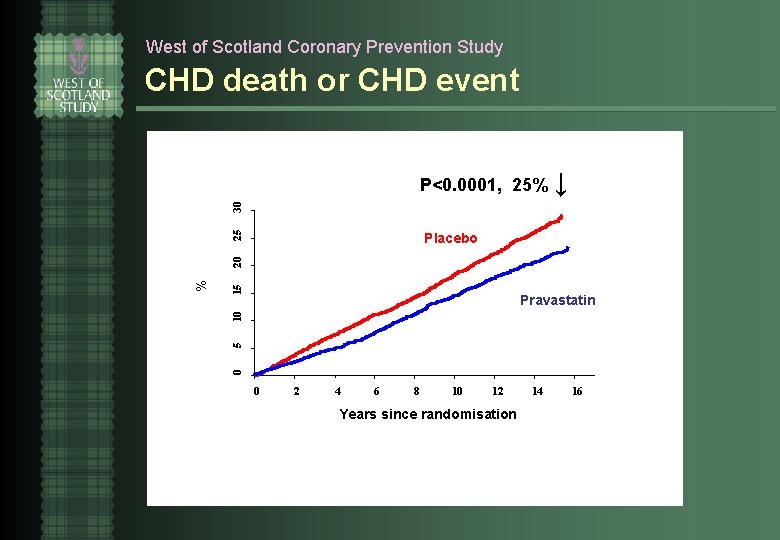
West of Scotland Coronary Prevention Study CHD death or CHD event P<0. 0001, 25% ↓ 25 30 P<0. 0001 15 10 Pravastatin 5 Placebo 0 % 20 Placebo 0 2 4 6 8 10 12 Years since randomisation 14 16

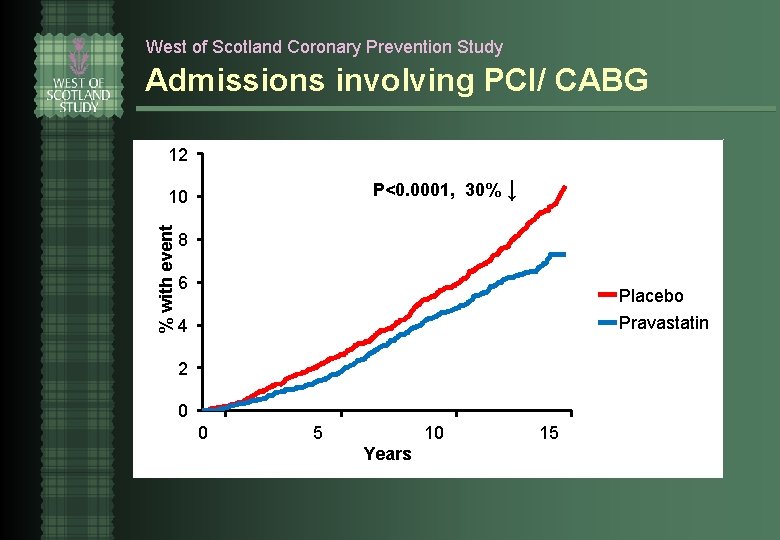
West of Scotland Coronary Prevention Study Admissions involving PCI/ CABG 12 P<0. 0001, 30% ↓ % with event 10 8 6 Placebo Pravastatin 4 2 0 0 5 10 Years 15

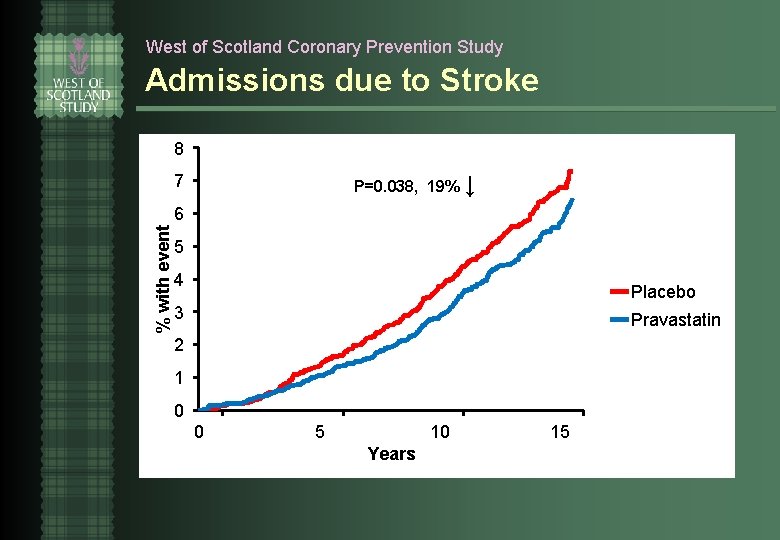
West of Scotland Coronary Prevention Study Admissions due to Stroke 8 P=0. 038, 19% ↓ 7 % with event 6 5 4 Placebo 3 Pravastatin 2 1 0 0 5 10 Years 15

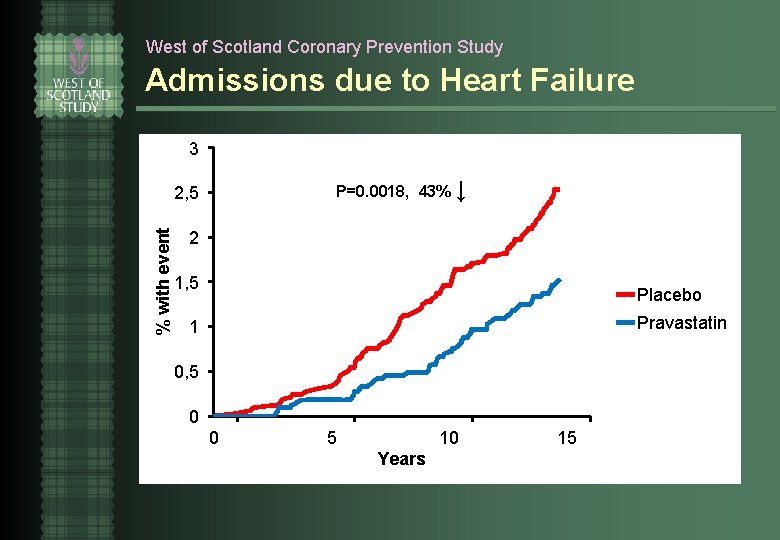
West of Scotland Coronary Prevention Study Admissions due to Heart Failure 3 P=0. 0018, 43% ↓ % with event 2, 5 2 1, 5 Placebo Pravastatin 1 0, 5 0 0 5 10 Years 15

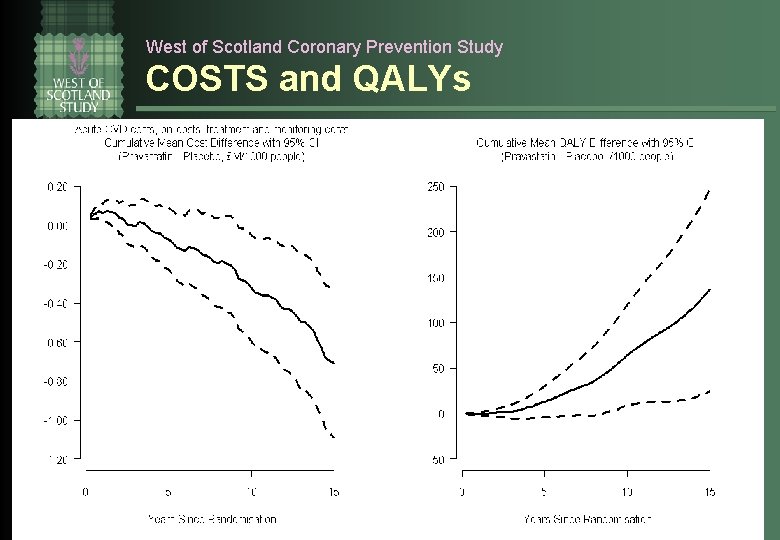
West of Scotland Coronary Prevention Study COSTS and QALYs

West of Scotland Coronary Prevention Study Benefits over 15 years treatment of 1000 patients would l save the UK NHS £ 710, 000 ( >$1 m) l gain 136 QALYs l prevent 1836 days in hospital l result in no excess in non-CV admissions/costs

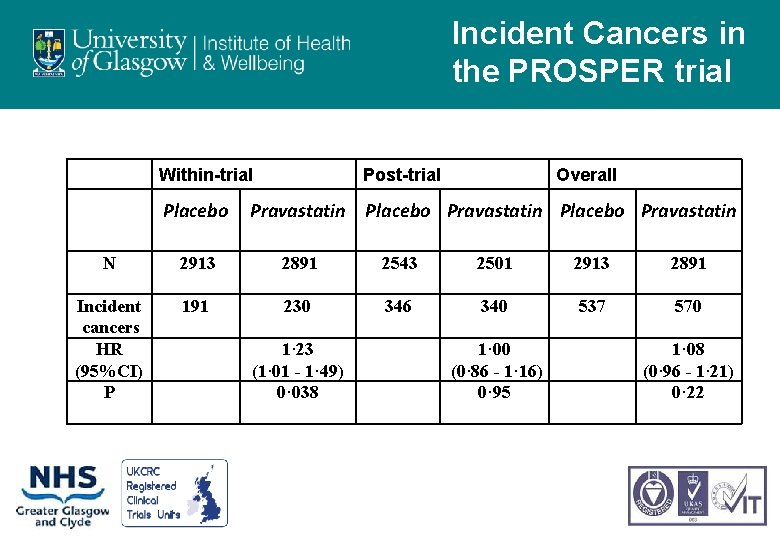
Incident Cancers in the PROSPER trial Within-trial Placebo Post-trial Overall Pravastatin Placebo Pravastatin N 2913 2891 2543 2501 2913 2891 Incident cancers HR (95%CI) P 191 230 346 340 537 570 1· 23 (1· 01 - 1· 49) 0· 038 1· 00 (0· 86 - 1· 16) 0· 95 1· 08 (0· 96 - 1· 21) 0· 22

Some challenges • Regulatory authorities – eg SAE reporting • Need for a national prescribing/dispensing dataset • Timeliness of data availability – challenge of acute studies • Incompleteness and quality of phenotype

What are industry doing • Electronic records for clinical research – EU IMI project – Aims to build a European platform to – support feasibility assessment – facilitate recruitment – facilitate data acquisition in follow-up

 Headgear tubes are routinely placed in which location
Headgear tubes are routinely placed in which location Electric foot mitts
Electric foot mitts Words images objects qualitative or quantitative
Words images objects qualitative or quantitative Data quality and data cleaning an overview
Data quality and data cleaning an overview Data quality and data cleaning an overview
Data quality and data cleaning an overview Data quality and data cleaning an overview
Data quality and data cleaning an overview Scientists recently discovered that rocks collected
Scientists recently discovered that rocks collected How are ballistics collected
How are ballistics collected Benefits of scanning in reading
Benefits of scanning in reading Pronoun
Pronoun Overview of education in health care
Overview of education in health care Master data services overview
Master data services overview Master data services overview
Master data services overview Chicago time
Chicago time An overview of data warehousing and olap technology
An overview of data warehousing and olap technology Trajectory data mining an overview
Trajectory data mining an overview Methodologies for cross-domain data fusion: an overview
Methodologies for cross-domain data fusion: an overview How you use ict today and how you will use it tomorrow
How you use ict today and how you will use it tomorrow Chapter 22 lesson 1
Chapter 22 lesson 1 Chapter 20 lesson 1 the health risks of tobacco use
Chapter 20 lesson 1 the health risks of tobacco use Chapter 20 lesson 1 the health risks of tobacco use
Chapter 20 lesson 1 the health risks of tobacco use Occupational health nurse roles and responsibilities
Occupational health nurse roles and responsibilities National programmes related to child health and welfare
National programmes related to child health and welfare Louisiana department of health and hospitals
Louisiana department of health and hospitals Difference between health education and propaganda
Difference between health education and propaganda Whole health circle of health
Whole health circle of health Health and social care component 3
Health and social care component 3 Health promotion world health organization
Health promotion world health organization University of central florida health care administration
University of central florida health care administration