New IVD Medical Device Act and Korea IVD







- Slides: 15

New IVD Medical Device Act and Korea IVD Industry Leejoon Kim RA QA manager Thermo Fisher Scientific, Korea July 16, 2019 The world leader in serving science

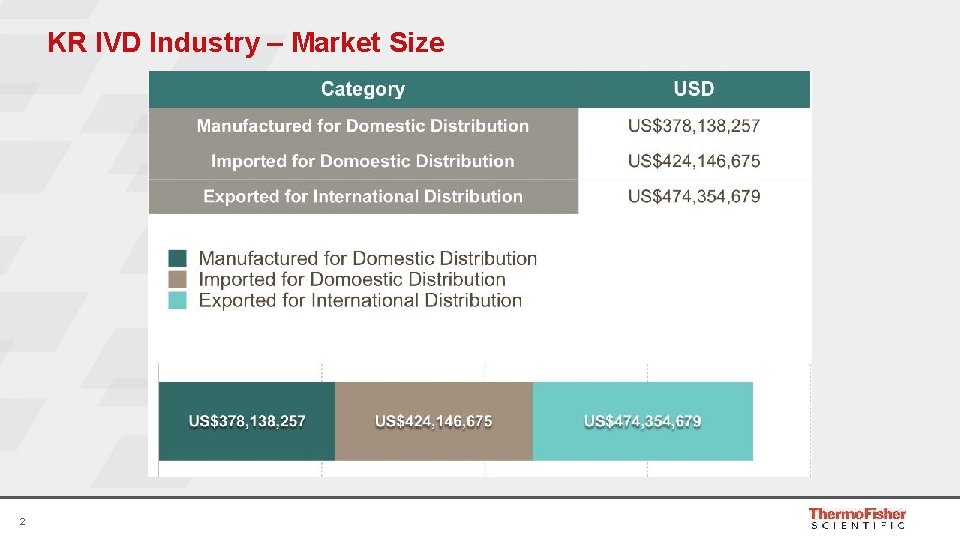
KR IVD Industry – Market Size 2

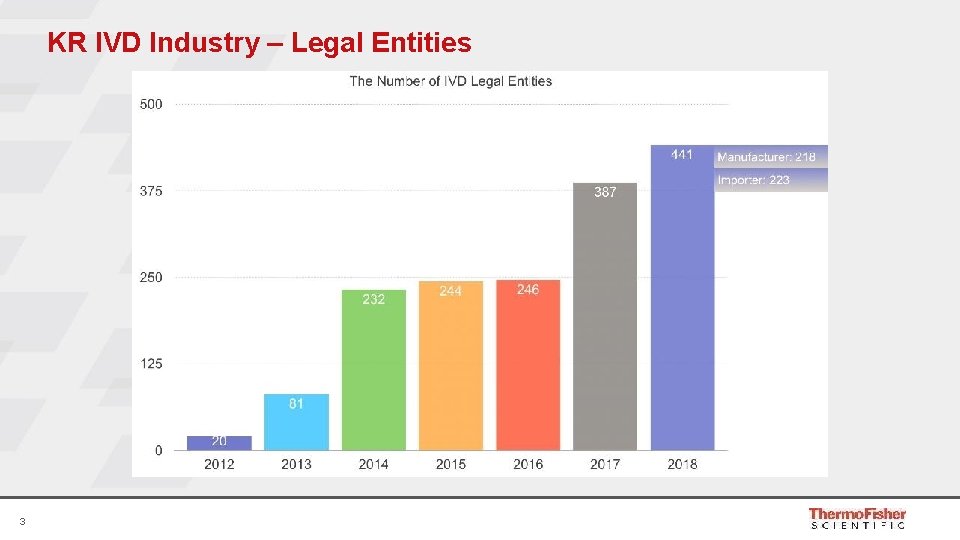
KR IVD Industry – Legal Entities 3

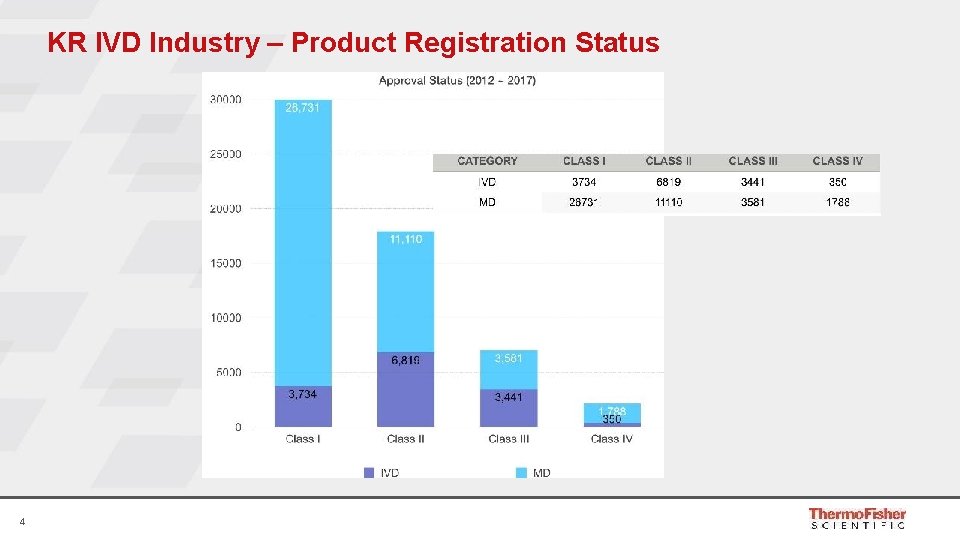
KR IVD Industry – Product Registration Status 4

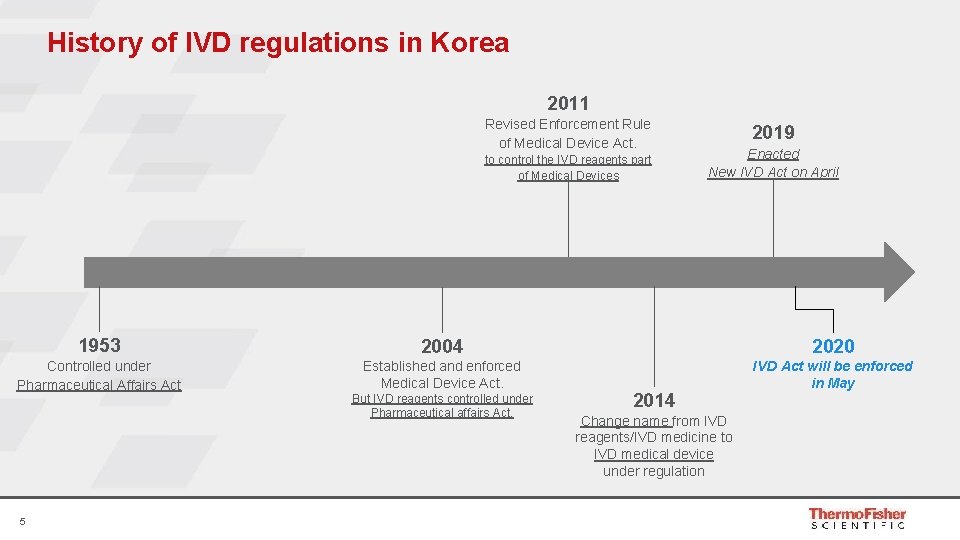
History of IVD regulations in Korea 2011 Revised Enforcement Rule of Medical Device Act. to control the IVD reagents part of Medical Devices 2019 Enacted New IVD Act on April 1953 2004 2020 Controlled under Pharmaceutical Affairs Act Established and enforced Medical Device Act. IVD Act will be enforced in May 5 But IVD reagents controlled under Pharmaceutical affairs Act. 2014 Change name from IVD reagents/IVD medicine to IVD medical device under regulation

Overview of new IVD Act in Korea • New IVD Act, separate from Medical Device Act • Enactment on April 30, 2019 • Enforcement from May 1, 2020 • The key contents of IVD Act are; ü Definition of the IVD medical devices including IVD reagents, equipment and software. ü Risk-based approach to classification ü Co-approval process for the companion diagnostics and the medicines ü Introduction of the clinical performance test system for the IVD devices ü Defined the requirements of Clinical Laboratory Accreditation from MFDS for hospitals or laboratories conducting IVD testing ü Negative system for the product change control 6

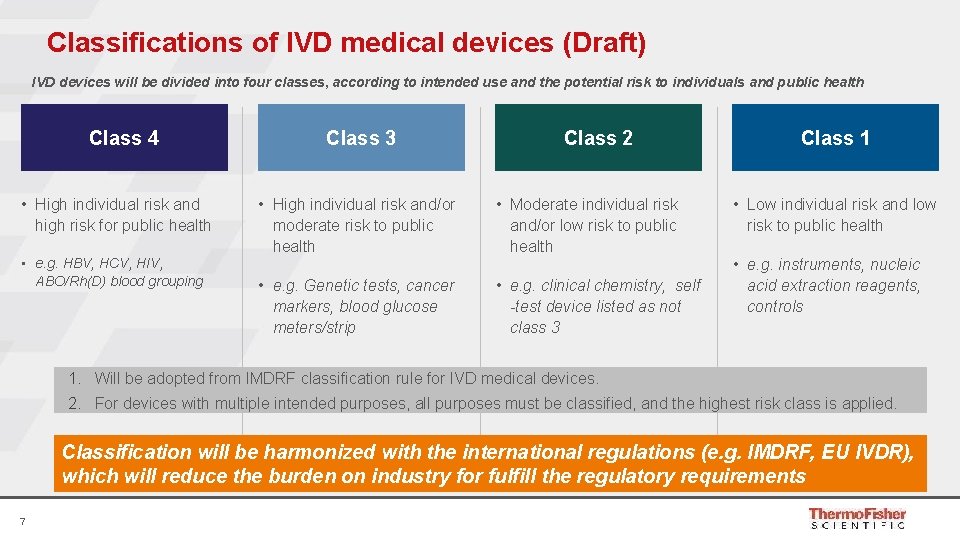
Classifications of IVD medical devices (Draft) IVD devices will be divided into four classes, according to intended use and the potential risk to individuals and public health Class 4 • High individual risk and high risk for public health • e. g. HBV, HCV, HIV, ABO/Rh(D) blood grouping Class 3 Class 2 • High individual risk and/or moderate risk to public health • Moderate individual risk and/or low risk to public health • e. g. Genetic tests, cancer markers, blood glucose meters/strip • e. g. clinical chemistry, self -test device listed as not class 3 Class 1 • Low individual risk and low risk to public health • e. g. instruments, nucleic acid extraction reagents, controls 1. Will be adopted from IMDRF classification rule for IVD medical devices. 2. For devices with multiple intended purposes, all purposes must be classified, and the highest risk class is applied. Classification will be harmonized with the international regulations (e. g. IMDRF, EU IVDR), which will reduce the burden on industry for fulfill the regulatory requirements 7

Clinical Laboratory Accreditation for IVD Testing ü For the use only within the applicable laboratories, it is intended for use of self-designed and configured IVD medical devices. ü Accredited laboratory shall obligate the requirements described in Enforcement Rule. ü NGS (Next-generation Sequencing) Clinical Laboratory Accreditation Already implemented before new IVD Act was enacted. ü Requirements of Accreditation of Clinical Lab 8 1. Quality management system of clinical laboratory 2. Proficiency of laboratory personnel 3. Performance evaluation of IVD testing

Clinical Laboratory Accreditation for IVD Testing (cont. ) ü Accredited clinical laboratory shall be following; 1. 2. 3. Reporting of annual test outcome Retention of the documents related to laboratory testing Compliance with quality management system ü Guidelines for NGS Clinical Laboratory Accreditation published by MFDS § NGS clinical laboratory Accreditation (Revised on Jun. 01, 2017) § Performance evaluation of NGS in vitro diagnostic medical devices (Revised on Jul. 31, 2018) § NGS Clinical Laboratory Accreditation for the field of testing • Noninvasive prenatal test(NIPT) (Revised on Feb. 7, 2018) • Germline (Revised on Feb. 7, 2018) • Somatic (Revised on Feb. 7, 2018) Detailed regulatory requirements and the scope of clinical laboratory accreditation in the field of IVD test Will be confirmed in the Enforcement Decree and the Enforcement Rules of IVD law. 9

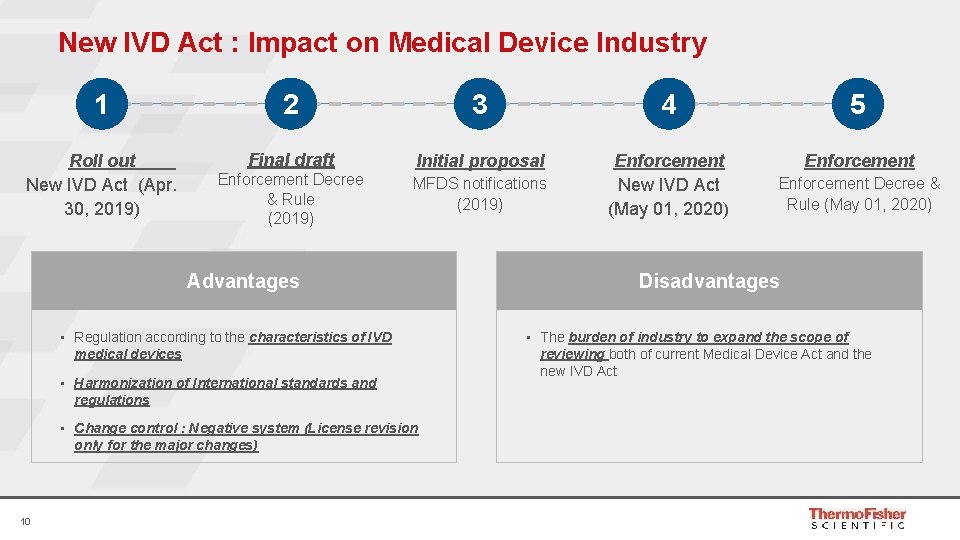
New IVD Act : Impact on Medical Device Industry 1 2 3 4 5 Roll out New IVD Act (Apr. 30, 2019) Final draft Initial proposal Enforcement New IVD Act (May 01, 2020) Enforcement Decree & Rule (2019) MFDS notifications (2019) Advantages • Regulation according to the characteristics of IVD medical devices • Harmonization of International standards and regulations • Change control : Negative system (License revision only for the major changes) 10 Enforcement Decree & Rule (May 01, 2020) Disadvantages • The burden of industry to expand the scope of reviewing both of current Medical Device Act and the new IVD Act

Act on Nurturing Medical Device Industry and Supporting Innovative Medical Devices (short title: Medical Devices Industry Act) • Overview • Enactment on April 30, 2019, enforcement from May 1, 2020 • To support medial devices industry and accelerate the commercialization of innovative medical devices • The key contents of Medical Device Industry Act are; ü Certification and the support of innovative medical devices companies (e. g. priority of participation of national R&D project, reduction of taxes, exception of research facility, exemption from various charges, etc. ) ü Fast track of the product approvals ü Exemption of some documents for the innovative medical software ü Support for clinical trial ü Comprehensive support center for the medical devices industry 11

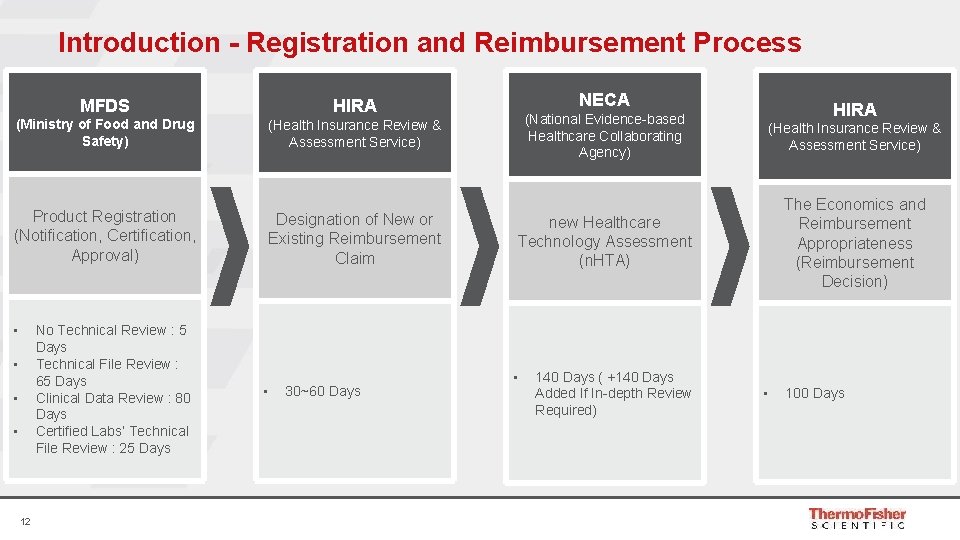
Introduction - Registration and Reimbursement Process MFDS HIRA (Ministry of Food and Drug Safety) (Health Insurance Review & Assessment Service) Product Registration (Notification, Certification, Approval) • No Technical Review : 5 Days Technical File Review : 65 Days Clinical Data Review : 80 Days Certified Labs’ Technical File Review : 25 Days • • • 12 Designation of New or Existing Reimbursement Claim • 30~60 Days NECA HIRA (National Evidence-based Healthcare Collaborating Agency) (Health Insurance Review & Assessment Service) new Healthcare Technology Assessment (n. HTA) The Economics and Reimbursement Appropriateness (Reimbursement Decision) • 140 Days ( +140 Days Added If In-depth Review Required) • 100 Days

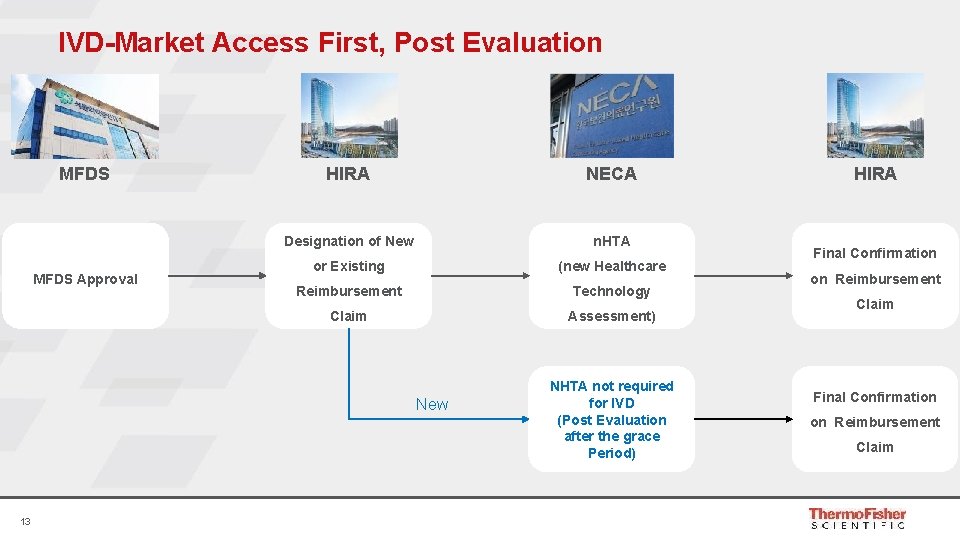
IVD-Market Access First, Post Evaluation MFDS Approval HIRA NECA Designation of New n. HTA or Existing (new Healthcare Reimbursement Technology Claim Assessment) New 13 NHTA not required for IVD (Post Evaluation after the grace Period) HIRA Final Confirmation on Reimbursement Claim

DTC (direct-to-consumer) Testing DTC Genetic testing controlled under Bioethics and Safety Act. (Not controlled by New IVD law) Accredited genetic testing can be provided to customer for 12 items & 46 genes. Ministry of Health and Welfare has been conducting a pilot project of DTC genetic testing service accreditation until September 2019. In pilot project of DTC accreditation, the testing items expanded up to 57. 14

Thank You 15
 Hardware output
Hardware output Ivd product development process
Ivd product development process Rudolf koch ivd
Rudolf koch ivd Companion diagnostic clinical trial
Companion diagnostic clinical trial Ivd 4 pint
Ivd 4 pint Ivd coating wiki
Ivd coating wiki Edma code
Edma code New era korea
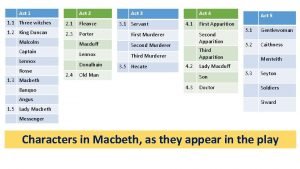
New era korea Macbeth act 3-5 summary
Macbeth act 3-5 summary Colonialism and development: korea, taiwan, and kwantung
Colonialism and development: korea, taiwan, and kwantung Difference between medical report and medical certificate
Difference between medical report and medical certificate A tagout device is preferable to using a lockout device.
A tagout device is preferable to using a lockout device. Input device dan output device
Input device dan output device Global medical device nomenclature
Global medical device nomenclature Medical device academy
Medical device academy Medical device security conference
Medical device security conference