New and Expanded Use of Vaccines Influenza Meningococcal







- Slides: 13

New and Expanded Use of Vaccines: Influenza & Meningococcal Vaccines H. Cody Meissner, M. D. Tufts-New England Medical Center 38 th National Immunization Conference Nashville, TN May 13, 2004

Influenza Virus

Addition to TIV Influenza Vaccine Recommendations • Immunization of healthy children between 6 -24 months of age • Immunization of household contacts, out -of-home caregivers and health care providers of children <24 months • Immunization of close contacts of children <6 months particularly important

Routine Influenza Immunization at 6 -24 Months • Influenza disease burden in children: • Attack rate 15 -42% in preschool & school age • Outpatient visits attributable to influenza 6 -29/100 children • 3 -5% of children experience AOM • 10 -30% increase in antibiotic courses • May be associated with severe pneumococcal or staphylococcal pneumonia • Hospitalization rate of 1. 9 -4. 8/100, 000 population

Influenza Vaccines & Thimerosal • No scientific evidence indicates that thimerosal in vaccines leads to serious adverse events • LAIV does not contain thimerosal • Thimerosal containing TIV vaccine 25 µg/0. 5 m. L & reduced thimerosal vaccine <1 µg/0. 5 m. L • Benefits of influenza vaccination outweigh theoretical risk for thimerosal exposure • Either vaccine preparation may be administered to any person for whom immunization is recommended

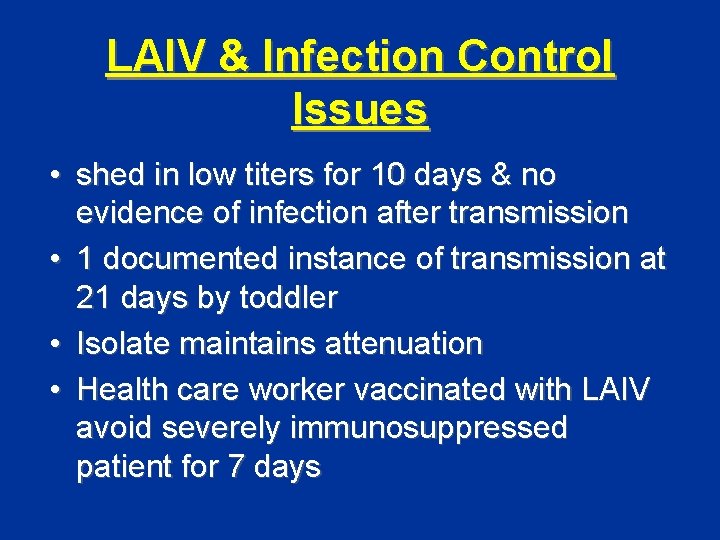
LAIV & Infection Control Issues • shed in low titers for 10 days & no evidence of infection after transmission • 1 documented instance of transmission at 21 days by toddler • Isolate maintains attenuation • Health care worker vaccinated with LAIV avoid severely immunosuppressed patient for 7 days

Composition of 2004 -05 Influenza Vaccines 1. A/New Caledonia/20/99(H 1 N 1)-like 2. A/Fujian/411/2002/(H 3 N 2)-like 3. B/Shanghai/361/2002 -like

Outbreaks of Avian Influenza A in Poultry in Asia & US • Influenza A(H 5 N 1) in Asia & Pakistan • 22 deaths among 32 human cases • inefficient person to person transfer • contact with bird saliva, nasal secretions, feces • resistant to amantadine, rimantadine • Influenza A(H 7 N 2) • poultry flocks in US • generally does not infect humans

Influenza Questions • When will the next influenza pandemic occur? – three in the 20 th Century • Universal immunization? – Advantages – Simple message – Fixed market – Disadvantages – Expense – Logistics

Meningococcal Disease • 2000 -3000 cases reported each year • Overall rate ~ 1/100, 000 population • Highest age-specific rates are among infants and young children • Vaccine serotypes A, C, Y, W-135 account for ~65% of infections in US • Vaccine recommended for military recruits, asplenia, terminal complement deficiency, some travelers, consider for college students

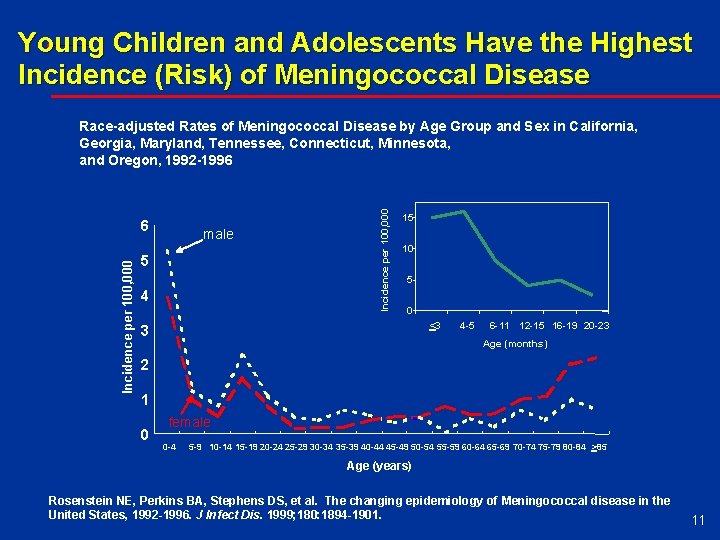
Young Children and Adolescents Have the Highest Incidence (Risk) of Meningococcal Disease Incidence per 100, 000 6 male 5 4 Incidence per 100, 000 Race-adjusted Rates of Meningococcal Disease by Age Group and Sex in California, Georgia, Maryland, Tennessee, Connecticut, Minnesota, and Oregon, 1992 -1996 15 10 5 0 <3 3 4 -5 6 -11 12 -15 16 -19 20 -23 Age (months) 2 1 0 female 0 -4 5 -9 10 -14 15 -19 20 -24 25 -29 30 -34 35 -39 40 -44 45 -49 50 -54 55 -59 60 -64 65 -69 70 -74 75 -79 80 -84 >85 Age (years) Rosenstein NE, Perkins BA, Stephens DS, et al. The changing epidemiology of Meningococcal disease in the United States, 1992 -1996. J Infect Dis. 1999; 180: 1894 -1901. 11

Reasons the Meningococcal Polysaccharide Vaccine Not Routinely Recommended 1. Polysaccharide vaccine not effective <24 months 2. Does not contain serogroup B 3. Induced immunity is short-lived 4. Conjugate vaccine available soon 5. Polysaccharide vaccine unlikely to be cost effective NEJM 2003; 349: 2353

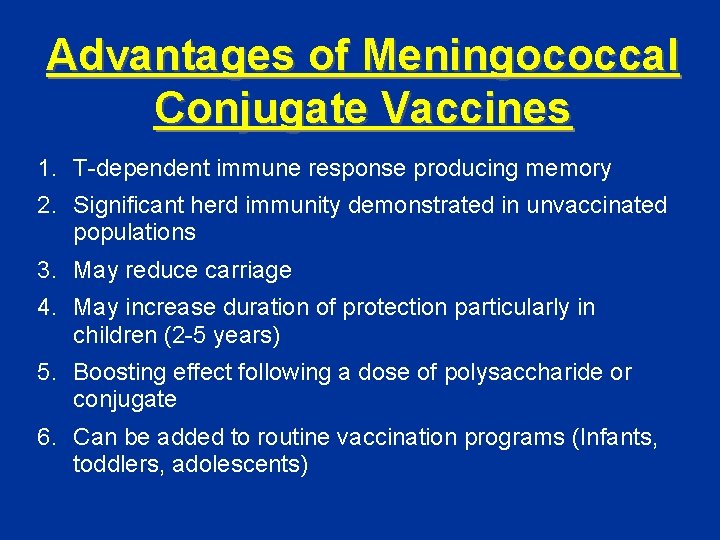
Advantages of Meningococcal Conjugate Vaccines 1. T-dependent immune response producing memory 2. Significant herd immunity demonstrated in unvaccinated populations 3. May reduce carriage 4. May increase duration of protection particularly in children (2 -5 years) 5. Boosting effect following a dose of polysaccharide or conjugate 6. Can be added to routine vaccination programs (Infants, toddlers, adolescents)
 Invasive meningococcal disease
Invasive meningococcal disease Virulent
Virulent Edible vaccines pros and cons
Edible vaccines pros and cons What is the difference between vaccination and inoculation
What is the difference between vaccination and inoculation Global alliance for vaccines and immunization
Global alliance for vaccines and immunization Edible vaccines pros and cons
Edible vaccines pros and cons Glyphosate in vaccines
Glyphosate in vaccines Edible vaccines in pharmacognosy
Edible vaccines in pharmacognosy Stephanie seneff mit
Stephanie seneff mit Immune checkpoint inhibitors mechanism of action
Immune checkpoint inhibitors mechanism of action Tubertest idr
Tubertest idr Could vaccines breed viciousness
Could vaccines breed viciousness Hep b vaccines
Hep b vaccines Virulent
Virulent