Influenza and Influenza Vaccines Clinicians Outreach and Communication



















- Slides: 45

Influenza and Influenza Vaccines Clinician’s Outreach and Communication Activity 2007 -2008 Season

Influenza • Highly infectious viral illness • Epidemics reported since 16 th century • Virus first isolated in 1933 2

Influenza Virus Strains • Type A – moderate to severe illness – all age groups – humans and other animals • Type B – changes less rapidly than type A – milder epidemics – humans only – primarily affects children 3

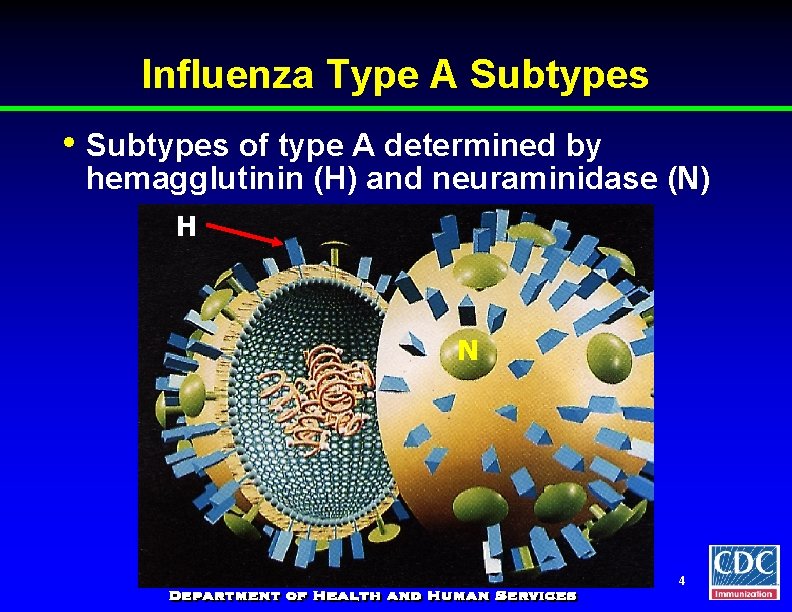
Influenza Type A Subtypes • Subtypes of type A determined by hemagglutinin (H) and neuraminidase (N) H N 4

Surface Antigens and Immunity • Immunity reduces likelihood of • • • infection and severity of disease Antibodies are specific to different types of surface antigens Changes in H and N allow virus to evade previously developed immune responses Antigenic changes: drift and shift 5

Influenza Antigenic Changes • Antigenic Drift – Minor change, same subtype – Caused by point mutations in gene – May result in epidemic • Example of antigenic drift – In 2003 -2004, A/Fujian/411/2002 -like (H 3 N 2) virus was dominant – A/California/7/2004 (H 3 N 2) began to circulate and became the dominant virus in 2005 6

Influenza Antigenic Changes • Antigenic Shift – Major change, new subtype – Caused by exchange of gene segments – May result in pandemic • Example of antigenic shift – H 2 N 2 virus circulated in 1957 -1967 – H 3 N 2 virus appeared in 1968 and completely replaced H 2 N 2 virus 7

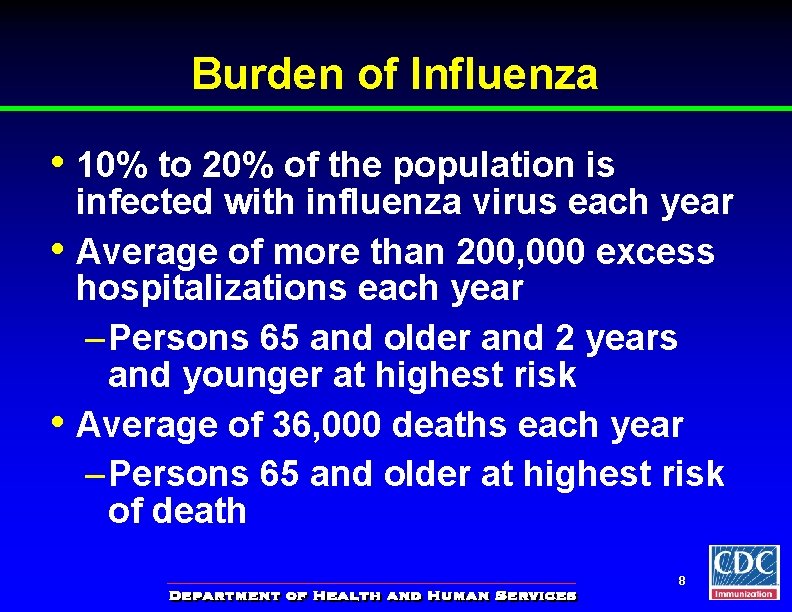
Burden of Influenza • 10% to 20% of the population is • • infected with influenza virus each year Average of more than 200, 000 excess hospitalizations each year – Persons 65 and older and 2 years and younger at highest risk Average of 36, 000 deaths each year – Persons 65 and older at highest risk of death 8

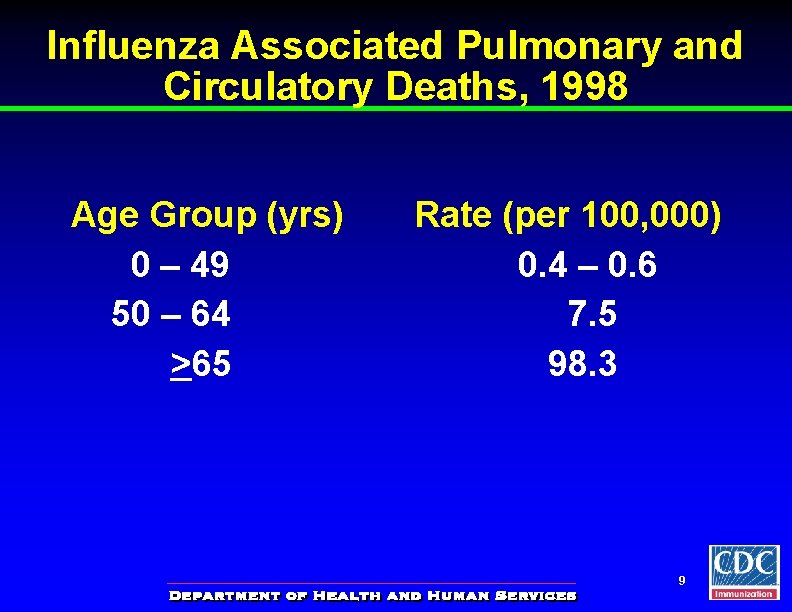
Influenza Associated Pulmonary and Circulatory Deaths, 1998 Age Group (yrs) 0 – 49 50 – 64 >65 Rate (per 100, 000) 0. 4 – 0. 6 7. 5 98. 3 9

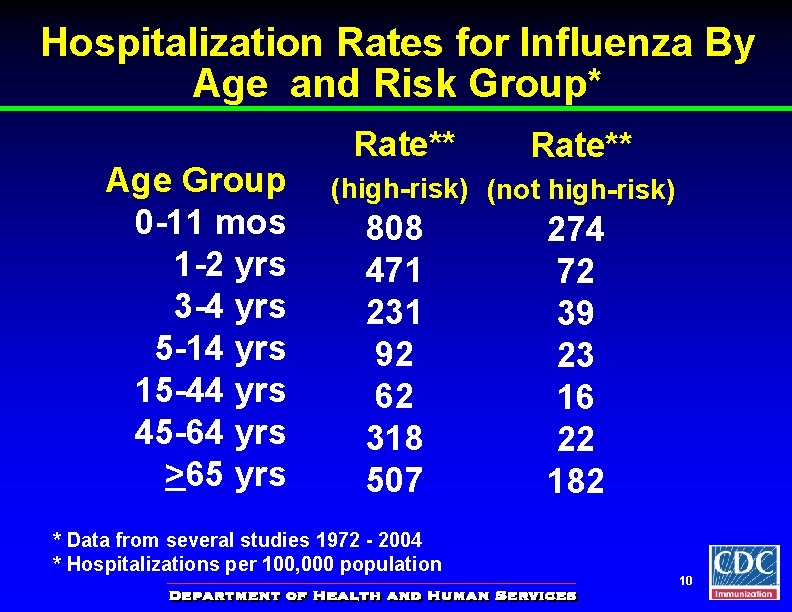
Hospitalization Rates for Influenza By Age and Risk Group* Age Group 0 -11 mos 1 -2 yrs 3 -4 yrs 5 -14 yrs 15 -44 yrs 45 -64 yrs >65 yrs Rate** (high-risk) (not high-risk) 808 471 231 92 62 318 507 * Data from several studies 1972 - 2004 * Hospitalizations per 100, 000 population 274 72 39 23 16 22 182 10

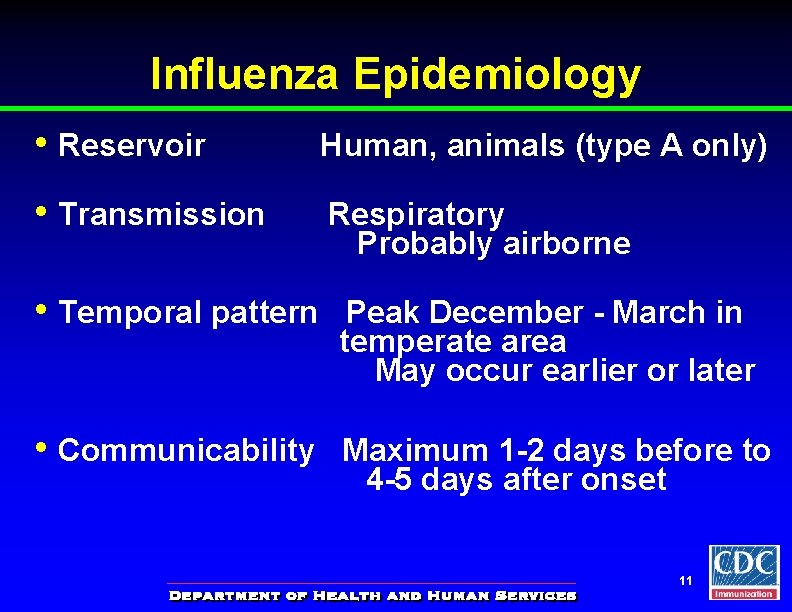
Influenza Epidemiology • Reservoir Human, animals (type A only) • Transmission Respiratory Probably airborne • Temporal pattern Peak December - March in temperate area May occur earlier or later • Communicability Maximum 1 -2 days before to 4 -5 days after onset 11

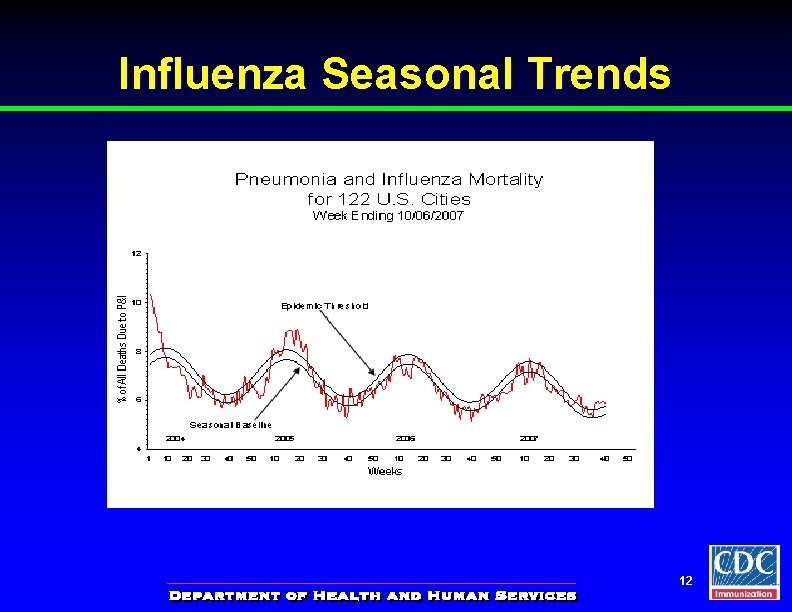
Influenza Seasonal Trends 12

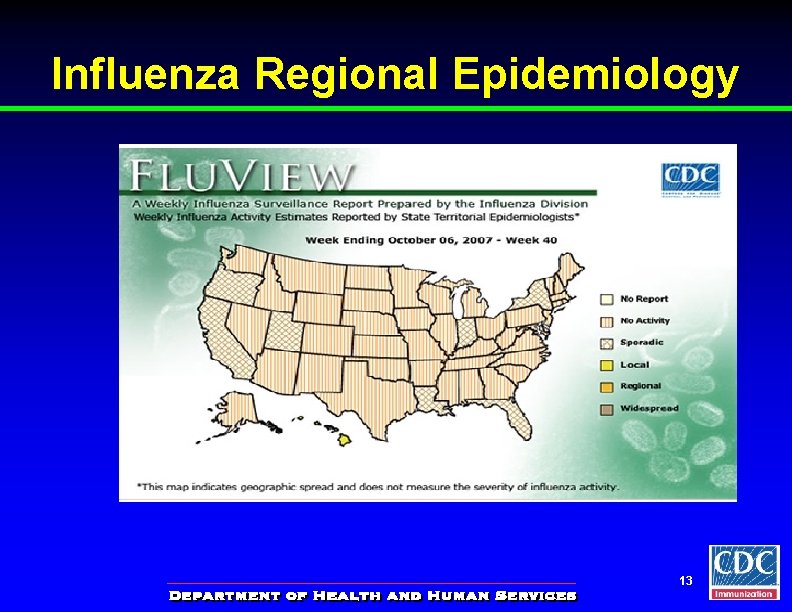
Influenza Regional Epidemiology 13

Influenza Vaccines • Inactivated subunit (TIV) – Intramuscular – Trivalent – Annual • Live attenuated vaccine (LAIV) – Intranasal – Trivalent – Annual 14

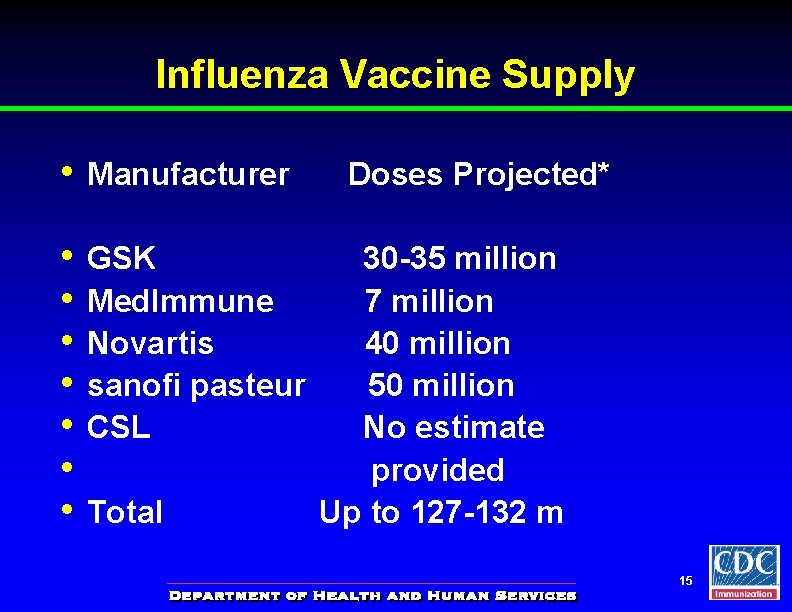
Influenza Vaccine Supply • Manufacturer • • GSK Med. Immune Novartis sanofi pasteur CSL Total Doses Projected* 30 -35 million 7 million 40 million 50 million No estimate provided Up to 127 -132 m 15

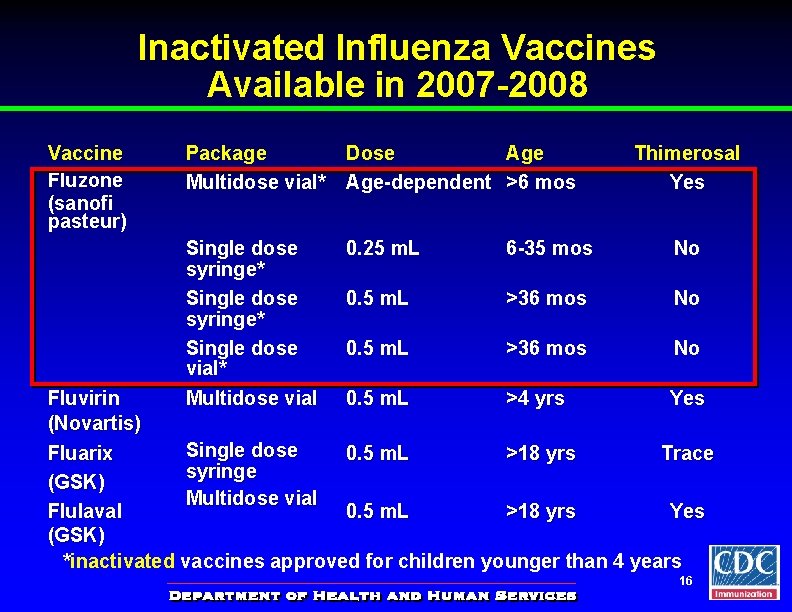
Inactivated Influenza Vaccines Available in 2007 -2008 Vaccine Fluzone (sanofi pasteur) Package Multidose vial* Dose Age-dependent >6 mos Thimerosal Yes Single dose syringe* Single dose vial* Multidose vial 0. 25 m. L 6 -35 mos No 0. 5 m. L >36 mos No Fluvirin 0. 5 m. L >4 yrs Yes (Novartis) Single dose Fluarix 0. 5 m. L >18 yrs Trace syringe (GSK) Multidose vial Flulaval 0. 5 m. L >18 yrs Yes (GSK) *inactivated vaccines approved for children younger than 4 years 16

17

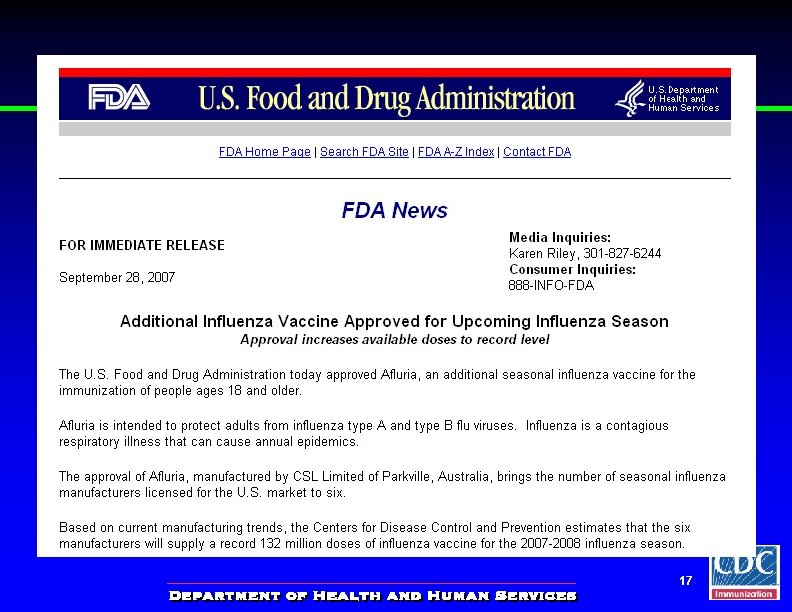
Afluria® Influenza Vaccine • Trivalent inactivated vaccine produced in hen’s eggs • Approved for persons 18 years and older • Available in – Preservative-free prefilled syringe – Multidose vial • Similar adverse reaction profile as other inactivated influenza vaccines 18

Why a Yearly Influenza Vaccination • Influenza vaccine expires June 30 each year • Antibodies wane during the year • Surface antigens drift and shift 19

2007 -2008 Influenza Vaccine • A/Wisconsin/67/2005 -like (H 3 N 2) • A/Solomon Islands/3/2006 -like (H 1 N 1) • B/Malaysia/2506/2004 -like (Victoria) 20

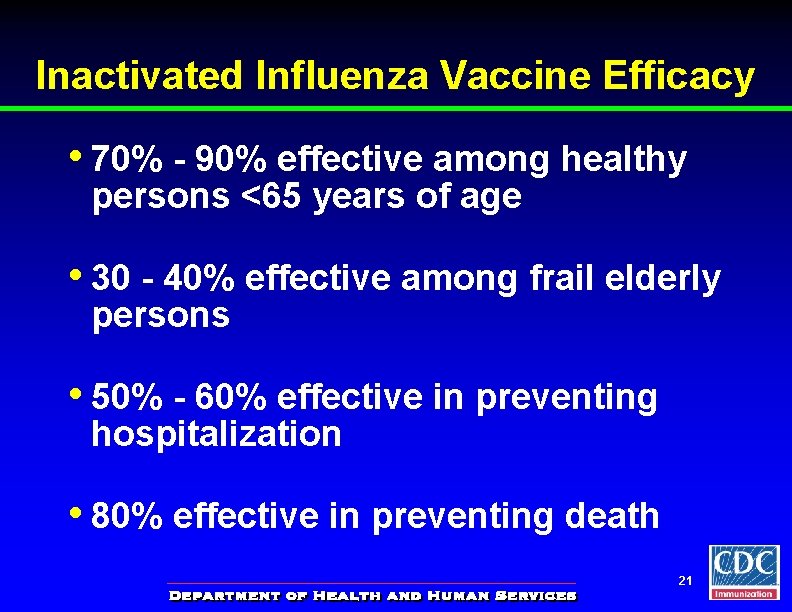
Inactivated Influenza Vaccine Efficacy • 70% - 90% effective among healthy persons <65 years of age • 30 - 40% effective among frail elderly persons • 50% - 60% effective in preventing hospitalization • 80% effective in preventing death 21

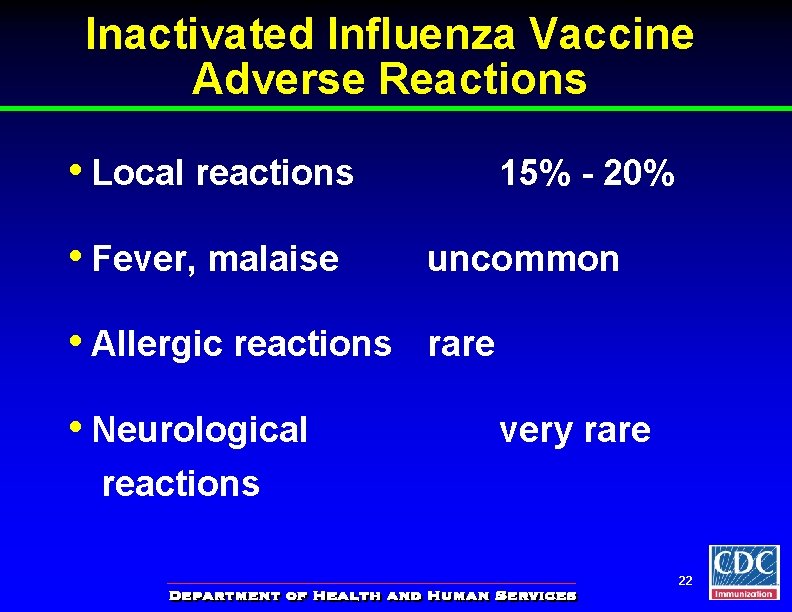
Inactivated Influenza Vaccine Adverse Reactions • Local reactions 15% - 20% • Fever, malaise uncommon • Allergic reactions rare • Neurological very rare reactions 22

Inactivated Influenza Vaccine Adverse Reactions • Inactivated influenza vaccine contains only noninfectious fragments of influenza virus • Inactivated influenza vaccine cannot cause influenza disease 23

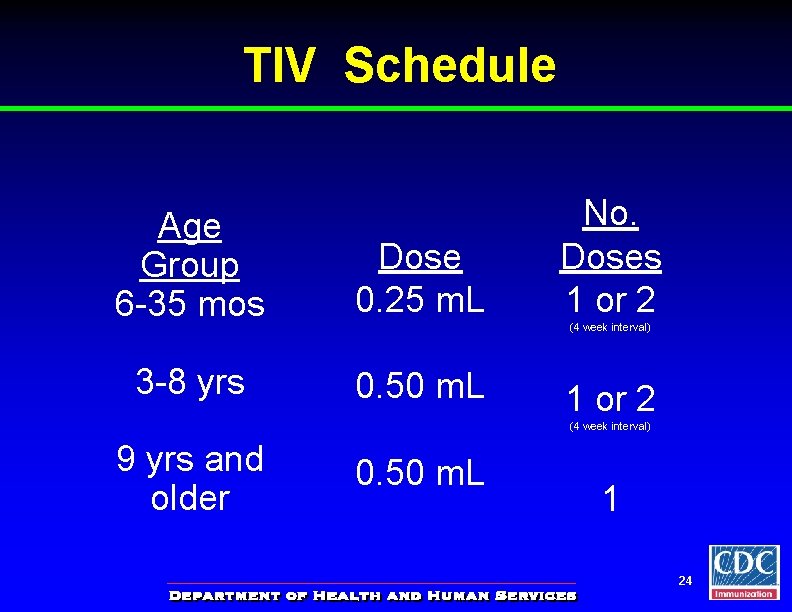
TIV Schedule Age Group 6 -35 mos Dose 0. 25 m. L 3 -8 yrs 0. 50 m. L No. Doses 1 or 2 (4 week interval) 9 yrs and older 0. 50 m. L 1 24

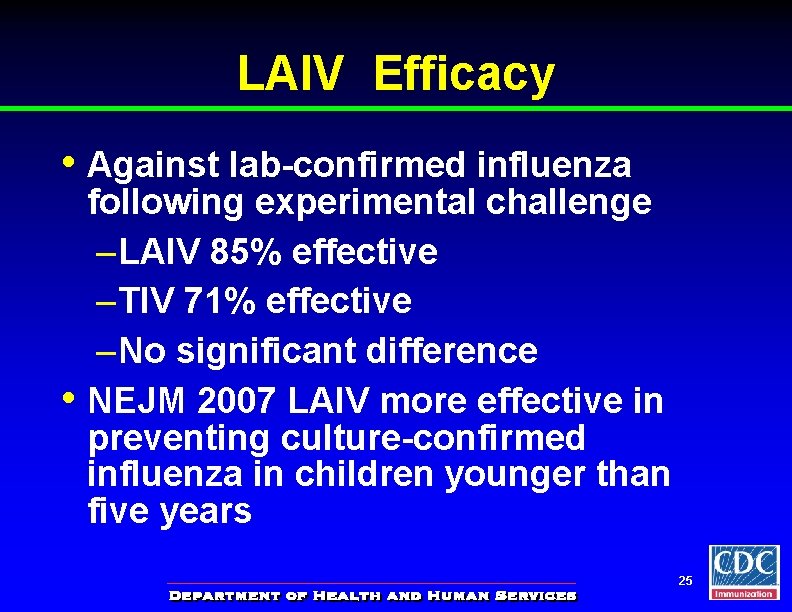
LAIV Efficacy • Against lab-confirmed influenza • following experimental challenge – LAIV 85% effective – TIV 71% effective – No significant difference NEJM 2007 LAIV more effective in preventing culture-confirmed influenza in children younger than five years 25

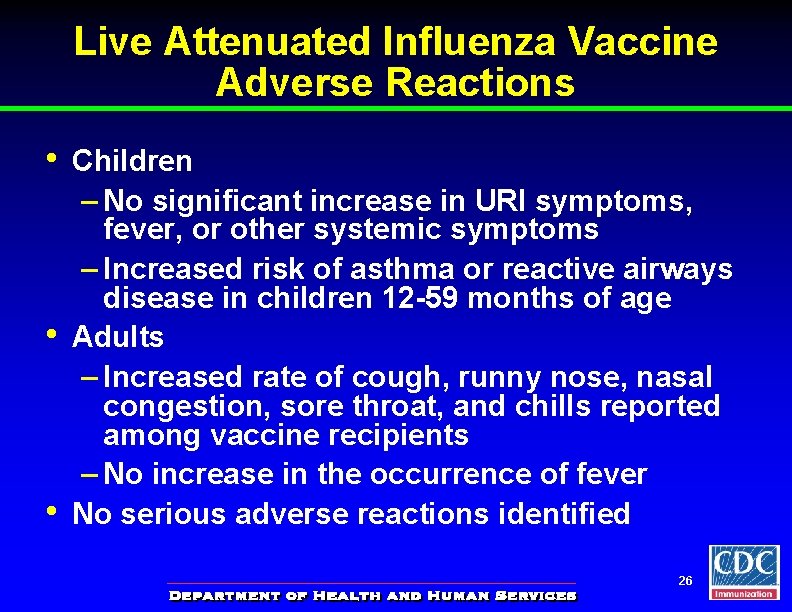
Live Attenuated Influenza Vaccine Adverse Reactions • • • Children – No significant increase in URI symptoms, fever, or other systemic symptoms – Increased risk of asthma or reactive airways disease in children 12 -59 months of age Adults – Increased rate of cough, runny nose, nasal congestion, sore throat, and chills reported among vaccine recipients – No increase in the occurrence of fever No serious adverse reactions identified 26

LAIV Indications • Healthy* persons 5 – 49 years of age – Close contacts of persons at high risk for complications of influenza (except severely immunosuppressed) – Persons who wish to reduce their own risk of influenza – Healthcare workers *Persons who do not have medical conditions that increase their risk for complications of influenza 27

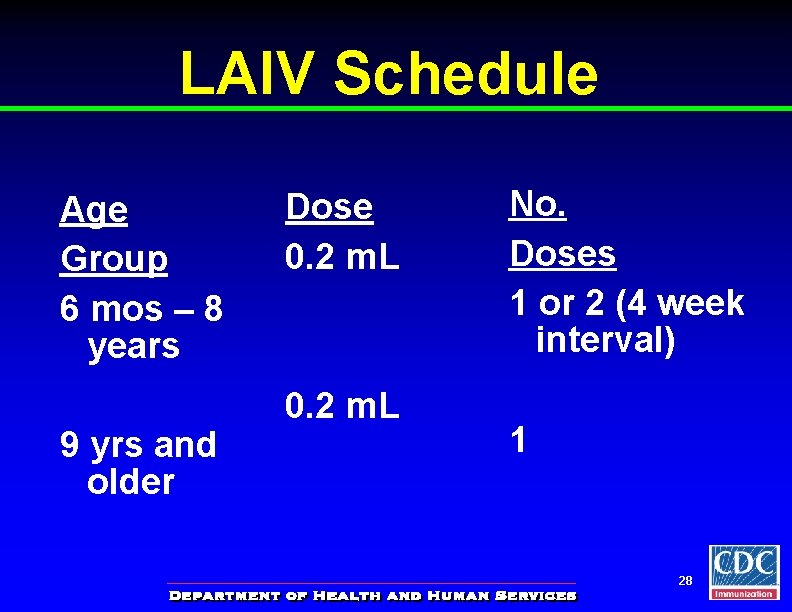
LAIV Schedule Age Group 6 mos – 8 years 9 yrs and older Dose 0. 2 m. L No. Doses 1 or 2 (4 week interval) 1 28

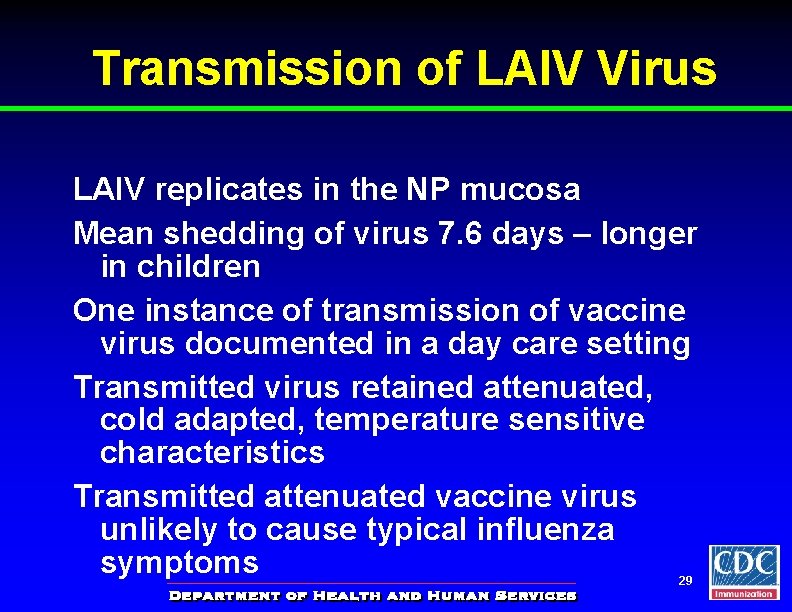
Transmission of LAIV Virus LAIV replicates in the NP mucosa Mean shedding of virus 7. 6 days – longer in children One instance of transmission of vaccine virus documented in a day care setting Transmitted virus retained attenuated, cold adapted, temperature sensitive characteristics Transmitted attenuated vaccine virus unlikely to cause typical influenza symptoms 29

Use of LAIV Among Healthcare Personnel No instances of transmission of LAIV have been reported in the U. S. ACIP recommends that LAIV can be given to eligible HCWs except those that care for severely immuno-suppressed persons (hospitalized and in isolation) No special precautions are required for HCWs who receive LAIV 30

LAIV Storage Must be stored at 35 - 46 degrees Fahrenheit Similar to TIV If inadvertently frozen, return to refrigerator 31

Influenza Season 2007 -2008 • • Recommended Groups for Vaccination Children 6 -59 months of age Healthy adults 50 years old and older Persons 5 – 49 years old at high risk for complications Pregnant women Residents of nursing homes Household contacts of persons at high risk for complications Health care workers 32

Influenza: High Risk for Complications • • • Birth through 59 months of age Adults 50 years old and older Chronic lung disease, asthma Chronic heart disease Metabolic diseases, e. g. diabetes Chronic renal disease High risk of aspiration Immunosuppression Pregnancy Chronic aspirin therapy: 18 years old and younger 33

HIV Infection and Inactivated Influenza Vaccine • Persons with HIV at higher risk for • • • complications of influenza TIV induces protective antibody titers in many HIV-infected persons Transient increase in HIV replication reported TIV will benefit many HIV-infected persons 34

Pregnancy and Inactivated Influenza Vaccine • Risk of hospitalization 4 times higher than nonpregnant women • Risk of complications comparable to nonpregnant women with high risk medical conditions • Vaccination (with TIV) recommended for all women who will be pregnant during the influenza season, regardless of gestational age 35

Influenza Vaccine Recommendations, 2007 -2008 • Immunization providers should administer influenza vaccine to any person who wishes to reduce the likelihood of becoming ill with influenza or transmitting influenza to others *Healthy persons 5 -49 years of age, including healthcare personnel may receive either TIV or LAIV 36

New Influenza Vaccine Recommendation 2007 -2008 • Children 6 months through 8 years • • being vaccinated for the first time should receive TWO doses Some children do not return for the second dose Beginning in influenza season 20072008 ACIP and AAP will recommend these children receive TWO doses the second vaccination year MMWR 2007; 56 (RR-6) 37

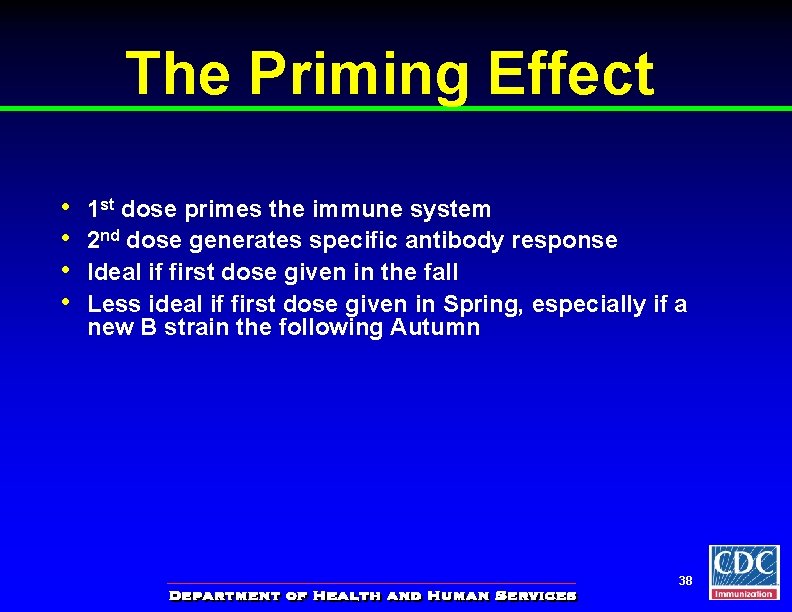
The Priming Effect • • 1 st dose primes the immune system 2 nd dose generates specific antibody response Ideal if first dose given in the fall Less ideal if first dose given in Spring, especially if a new B strain the following Autumn 38

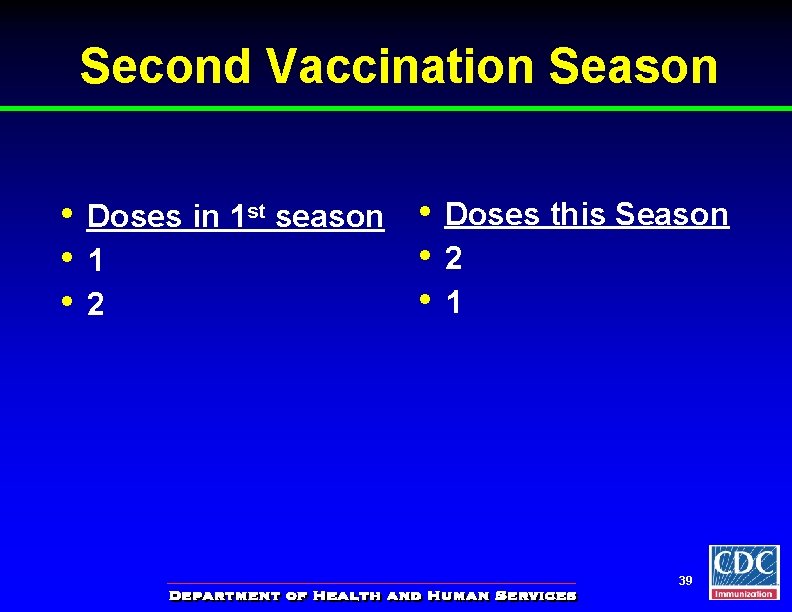
Second Vaccination Season • • • Doses in 1 st season 1 2 • • • Doses this Season 2 1 39

Mix and Match • • If two doses are indicated None are contraindicated Can mix and match TIV/LAIV Use interval of vaccine given first 40

The Magic of Ninth Birthday • • On or after ninth birthday Priming effect caused by natural infection thought to be significant Only one dose per season required Regardless of previous doses 41

Inactivated Influenza Vaccine Contraindications and Precautions • Contraindications • Severe allergic reaction to a vaccine component (e. g. , egg) or following a prior dose of vaccine • Precaution • Moderate or severe acute illness • History of Guillain-Barre within 6 weeks of prior dose 42

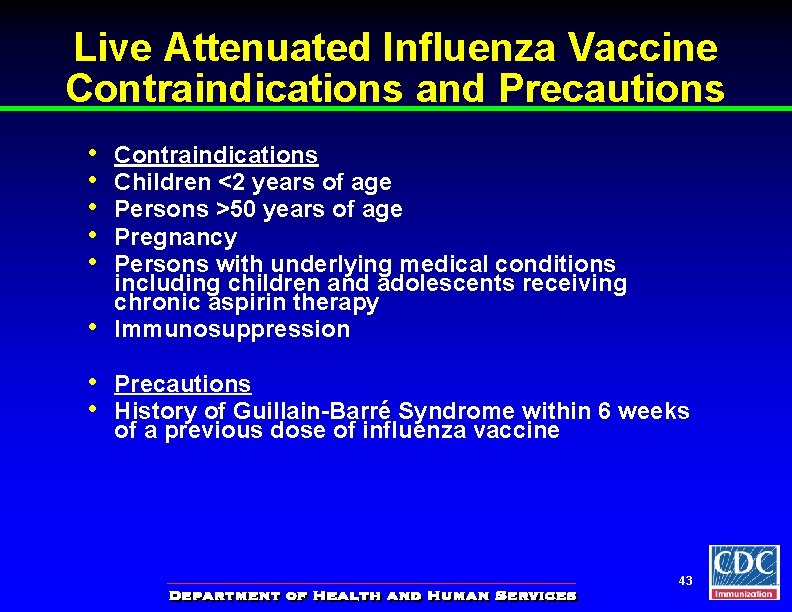
Live Attenuated Influenza Vaccine Contraindications and Precautions • • Contraindications Children <2 years of age Persons >50 years of age Pregnancy Persons with underlying medical conditions including children and adolescents receiving chronic aspirin therapy Immunosuppression Precautions History of Guillain-Barré Syndrome within 6 weeks of a previous dose of influenza vaccine 43

Influenza Antivirals • Use neuraminidase inhibitors • Oseltamavir: chemoprophylaxis and • treatment Zanamavir: treatment only • Avoid adamantanes due to resistance 44

Healthy Habits • • When Healthy: – Avoid close contact with those who are sick – Wash your hands often – Avoid touching your eyes, nose and mouth to decrease the spread of germs When Ill: – Cover your mouth and nose with a tissue (or upper sleeve) when you sneeze or cough – Stay home from work or school when you are sick 45