Microwave Spectra and Structure of CF 3 IPH

- Slides: 13

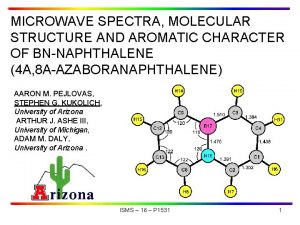
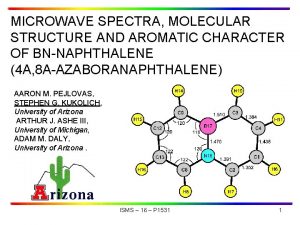
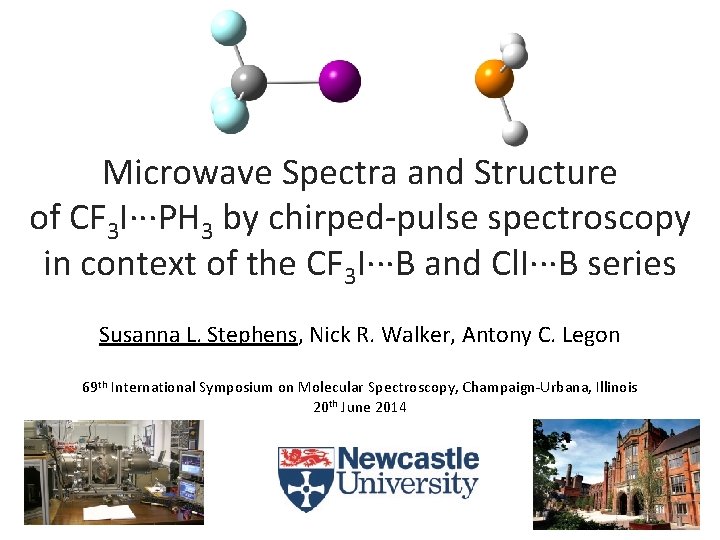
Microwave Spectra and Structure of CF 3 I∙∙∙PH 3 by chirped-pulse spectroscopy in context of the CF 3 I∙∙∙B and Cl. I∙∙∙B series Susanna L. Stephens, Nick R. Walker, Antony C. Legon 69 th International Symposium on Molecular Spectroscopy, Champaign-Urbana, Illinois 20 th June 2014

“A halogen bond R-X Y-Z occurs when there is evidence of a net attractive interaction between an electrophilic region on a halogen atom X belonging to a molecule or a molecular fragment R-X (where R can be another atom, including X, or a group of atoms) and a nucleophilic region of a molecule, or molecular fragment, Y-Z. ” The more of the following features of halogen bonding below that are satisfied the more reliable an identification of a halogen bond: 1) The length of the bond X Y will tend to be less than the sum of their Van de Waal radii of the atoms, X and Y. 2) The angle R-X Y will tend to be linear as the halogen will align with the lone-pair or π-system of the halogen acceptor. This angle may be perturbed slightly by other bonding effects elsewhere in the molecular system. 3) The covalent bond R-X will tend to increase upon bond to the halogen acceptor. 4) The strength of the halogen bonding will decrease when the electronegativity of X increases and the ability of R to withdraw electrons decreases. 5) Although the relative dependence of different bonding forces varies, the two primary bonding mechanisms are dispersion and electrostatic effects (which includes polarisation). 6) Electron density topology analysis tends to show a bond path X and Y and a bond critical point between X and Y. 7) Vibrational modes corresponding with the bond X Y are present upon bonding and vibrational modes in the Raman and IR regions of R-X and Y-Z are suitably shifted. 8) A characteristic shift to the blue is usually observed in the UV-visible spectrum of the halogen bond donor when the X Y bond is formed. 9) Typically the formation of the X Y bond will also change the characteristic nuclear magnetic resonance signals of R-X and Y-Z. 10) The halogen X may be involved in multiple halogen bonds. 11) The halogen bond may be included in reaction mechanisms including halogen transfer reactions. Gautam R. Desiraju, P. Shing Ho, Lars Kloo, Anthony C. Legon, Roberto Marquardt, Pierangelo Metrangolo, Peter A. Politzer, Giuseppe Resnati, and Kari Rissanen. Denition of the halogen bond, IUPAC Provisional Recommendation. http: //www. halogenbonding. eu/

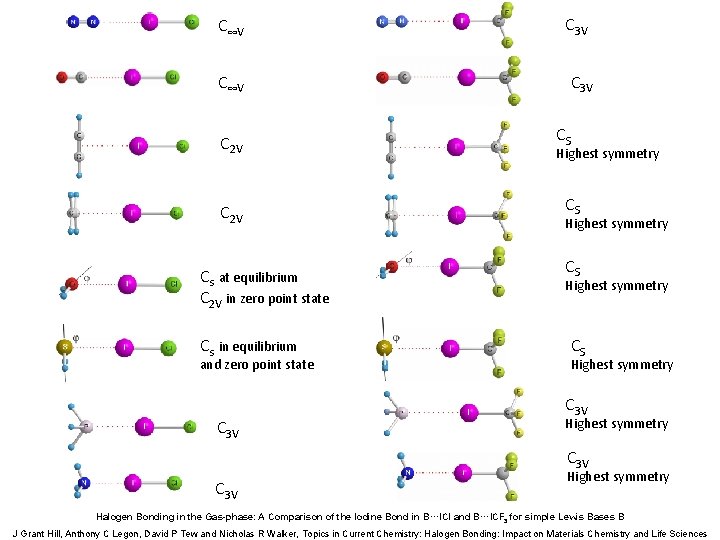
C∞V C 3 V C 2 V Cs at equilibrium C 2 V in zero point state Cs in equilibrium and zero point state C 3 V CS Highest symmetry C 3 V Highest symmetry Halogen Bonding in the Gas-phase: A Comparison of the Iodine Bond in B···ICl and B···ICF 3 for simple Lewis Bases B J Grant Hill, Anthony C Legon, David P Tew and Nicholas R Walker, Topics in Current Chemistry: Halogen Bonding: Impact on Materials Chemistry and Life Sciences

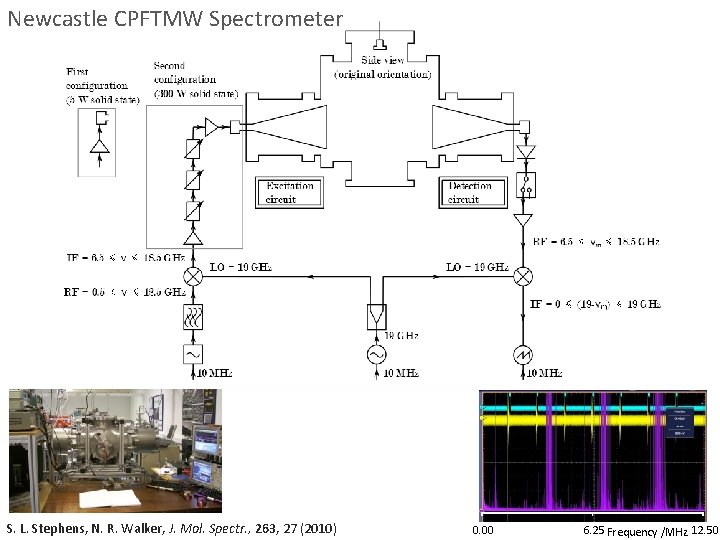
Newcastle CPFTMW Spectrometer S. L. Stephens, N. R. Walker, J. Mol. Spectr. , 263, 27 (2010) 0. 00 6. 25 Frequency /MHz 12. 50

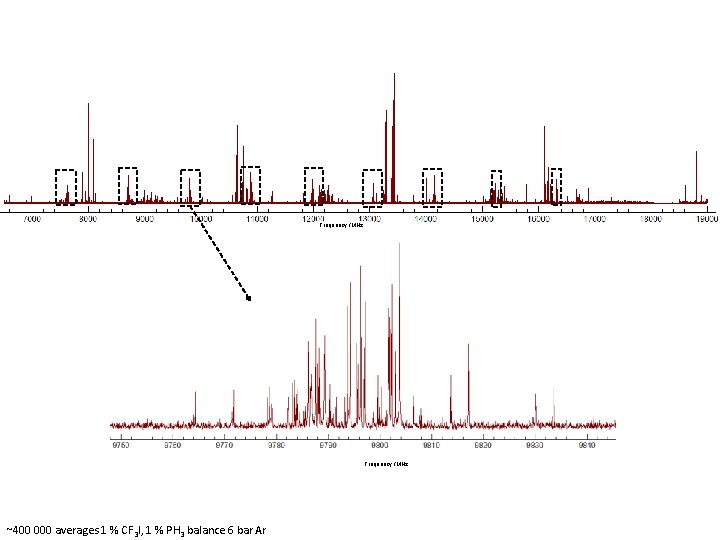
Frequency /MHz 107 Ag. I 109 Ag. I Frequency /MHz ~400 000 averages 1 % CF 3 I, 1 % PH 3 balance 6 bar Ar

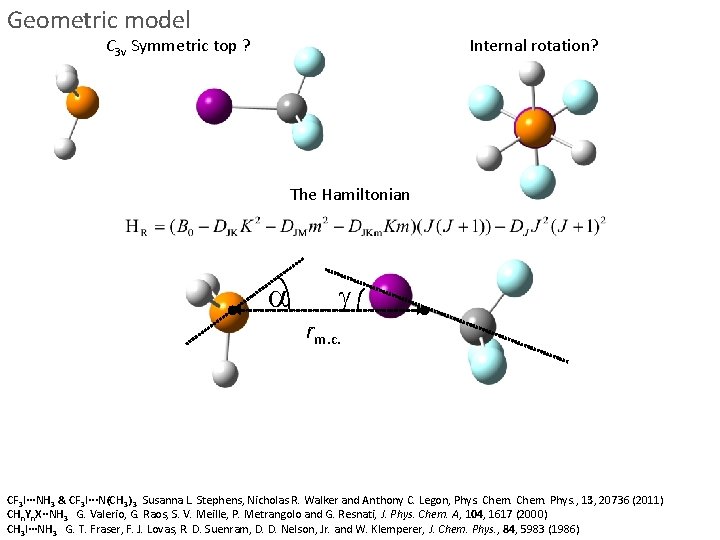
Geometric model C 3 v Symmetric top ? Internal rotation? The Hamiltonian a g rm. c. CF 3 I···NH 3 & CF 3 I···N(CH 3) 3 Susanna L. Stephens, Nicholas R. Walker and Anthony C. Legon, Phys. Chem. Phys. , 13, 20736 (2011) CHn. Yn. X··NH 3 G. Valerio, G. Raos, S. V. Meille, P. Metrangolo and G. Resnati, J. Phys. Chem. A, 104, 1617 (2000) CH 3 I···NH 3 G. T. Fraser, F. J. Lovas, R. D. Suenram, D. D. Nelson, Jr. and W. Klemperer, J. Chem. Phys. , 84, 5983 (1986)

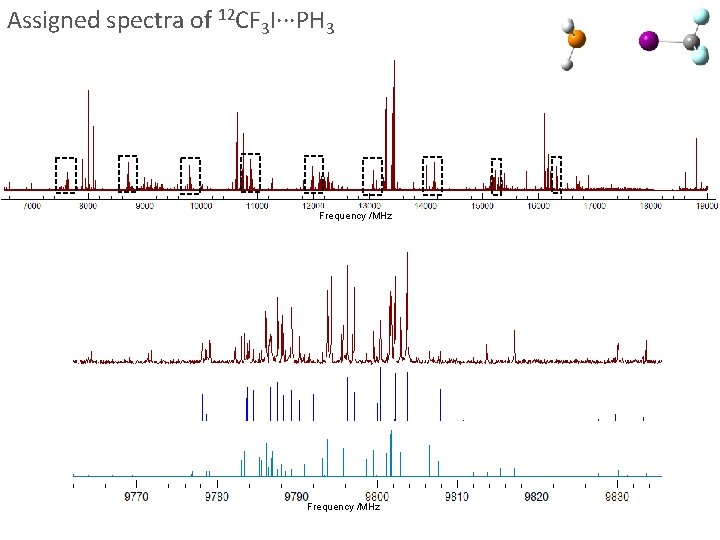
Assigned spectra of 12 CF 3 I∙∙∙PH 3 Frequency /MHz

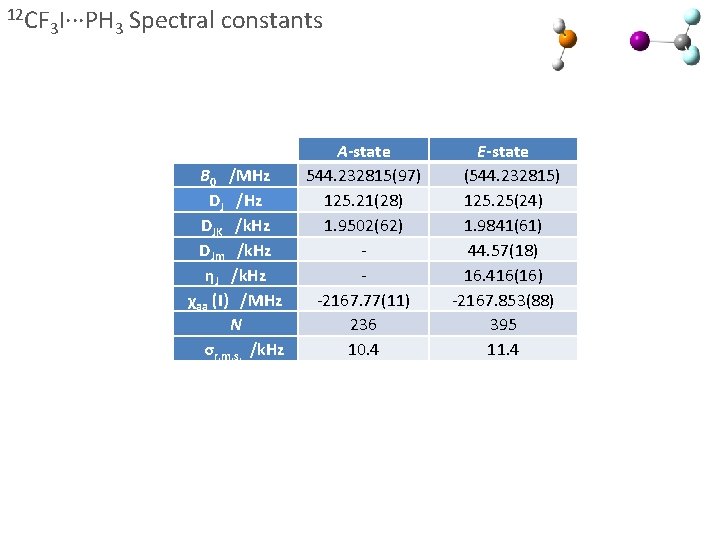
12 CF 3 I∙∙∙PH 3 Spectral constants B 0 /MHz DJ /Hz DJK /k. Hz DJm /k. Hz ηJ /k. Hz χaa (I) /MHz N σr. m. s. /k. Hz 84 Kr 57. 0 % 17. 3 % 82 Kr 11. 6 % 83 Kr 11. 5 % 86 Kr A-state 544. 232815(97) 125. 21(28) 1. 9502(62) -2167. 77(11) 236 10. 4 E-state (544. 232815) 125. 25(24) 1. 9841(61) 44. 57(18) 16. 416(16) -2167. 853(88) 395 11. 4

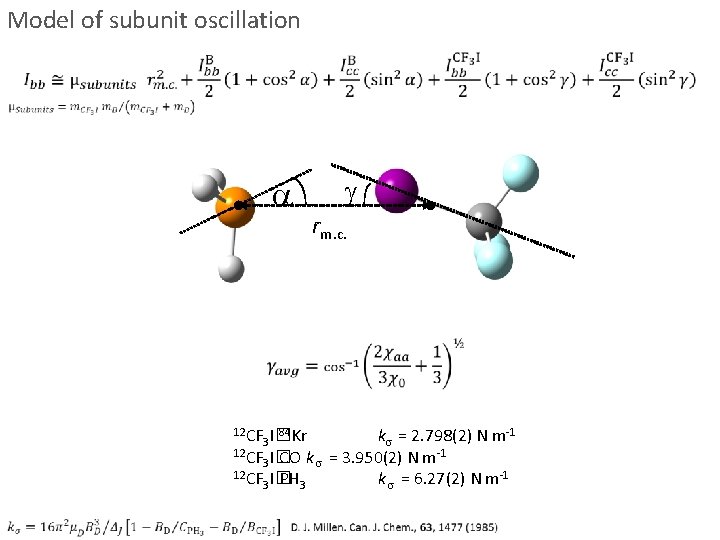
Model of subunit oscillation a g rm. c. 12 CF k = 2. 798(2) N m-1 12 CF I� -1 3 CO k = 3. 950(2) N m 12 CF I� k = 6. 27(2) N m-1 3 PH 3 84 3 I�Kr

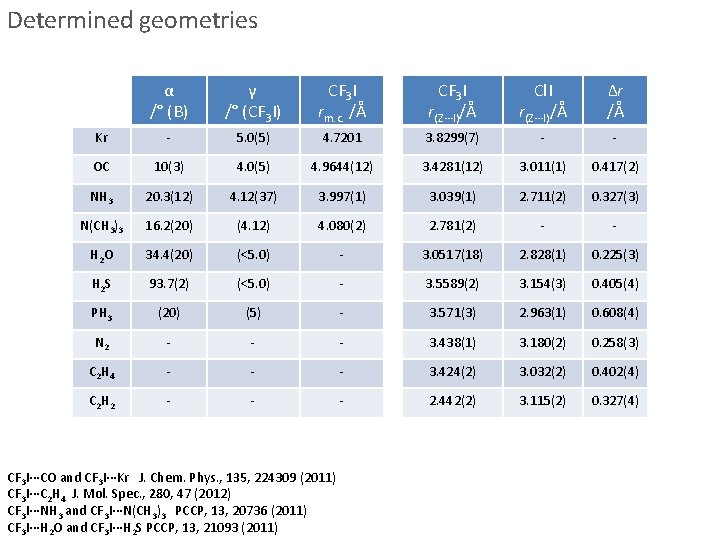
Determined geometries α /° (B) γ /° (CF 3 I) CF 3 I rm. c. /Å CF 3 I r(Z∙∙∙I)/Å Cl. I r(Z∙∙∙I)/Å Δr /Å Kr - 5. 0(5) 4. 7201 3. 8299(7) - - OC 10(3) 4. 0(5) 4. 9644(12) 3. 4281(12) 3. 011(1) 0. 417(2) NH 3 20. 3(12) 4. 12(37) 3. 997(1) 3. 039(1) 2. 711(2) 0. 327(3) N(CH 3)3 16. 2(20) (4. 12) 4. 080(2) 2. 781(2) - - H 2 O 34. 4(20) (<5. 0) - 3. 0517(18) 2. 828(1) 0. 225(3) H 2 S 93. 7(2) (<5. 0) - 3. 5589(2) 3. 154(3) 0. 405(4) PH 3 (20) (5) - 3. 571(3) 2. 963(1) 0. 608(4) N 2 - - - 3. 438(1) 3. 180(2) 0. 258(3) C 2 H 4 - - - 3. 424(2) 3. 032(2) 0. 402(4) C 2 H 2 - - - 2. 442(2) 3. 115(2) 0. 327(4) CF 3 I∙∙∙CO and CF 3 I∙∙∙Kr J. Chem. Phys. , 135, 224309 (2011) CF 3 I∙∙∙C 2 H 4 J. Mol. Spec. , 280, 47 (2012) CF 3 I∙∙∙NH 3 and CF 3 I∙∙∙N(CH 3)3 PCCP, 13, 20736 (2011) CF 3 I∙∙∙H 2 O and CF 3 I∙∙∙H 2 S PCCP, 13, 21093 (2011)

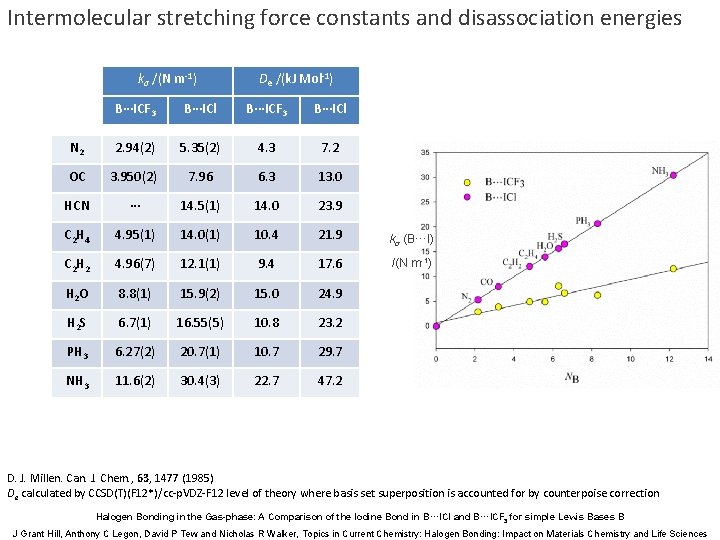
Intermolecular stretching force constants and disassociation energies kσ /(N m-1) De /(k. J Mol-1) B∙∙∙ICF 3 B∙∙∙ICl N 2 2. 94(2) 5. 35(2) 4. 3 7. 2 OC 3. 950(2) 7. 96 6. 3 13. 0 HCN ∙∙∙ 14. 5(1) 14. 0 23. 9 C 2 H 4 4. 95(1) 14. 0(1) 10. 4 21. 9 kσ (B···I) C 2 H 2 4. 96(7) 12. 1(1) 9. 4 17. 6 /(N m-1) H 2 O 8. 8(1) 15. 9(2) 15. 0 24. 9 H 2 S 6. 7(1) 16. 55(5) 10. 8 23. 2 PH 3 6. 27(2) 20. 7(1) 10. 7 29. 7 NH 3 11. 6(2) 30. 4(3) 22. 7 47. 2 D. J. Millen. Can. J. Chem. , 63, 1477 (1985) De calculated by CCSD(T)(F 12*)/cc-p. VDZ-F 12 level of theory where basis set superposition is accounted for by counterpoise correction Halogen Bonding in the Gas-phase: A Comparison of the Iodine Bond in B···ICl and B···ICF 3 for simple Lewis Bases B J Grant Hill, Anthony C Legon, David P Tew and Nicholas R Walker, Topics in Current Chemistry: Halogen Bonding: Impact on Materials Chemistry and Life Sciences

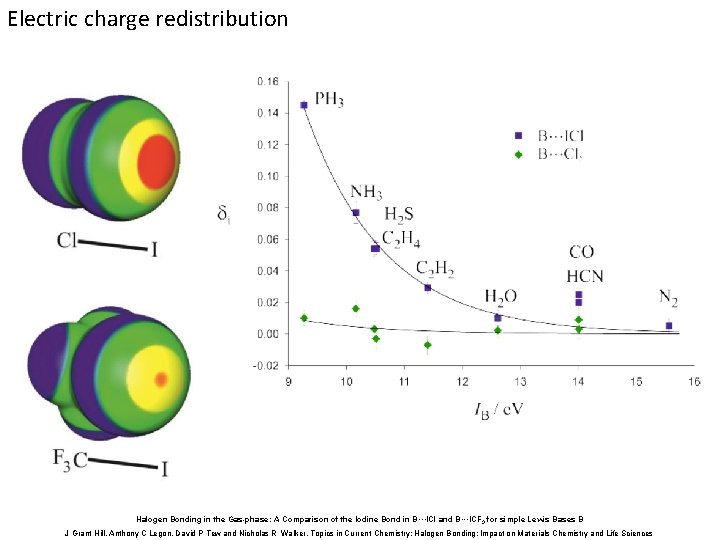
Electric charge redistribution Halogen Bonding in the Gas-phase: A Comparison of the Iodine Bond in B···ICl and B···ICF 3 for simple Lewis Bases B J Grant Hill, Anthony C Legon, David P Tew and Nicholas R Walker, Topics in Current Chemistry: Halogen Bonding: Impact on Materials Chemistry and Life Sciences

Acknowledgements University of Newcastle Nick Walker Daniel Zaleski David Hird Dror Bittner University of Bristol Tony C. Legon David Tew Colin M. Western Halogen Bonding in the Gas-phase: A Comparison of the Iodine Bond in B···ICl and B···ICF 3 for simple Lewis Bases B J Grant Hill, Anthony C Legon, David P Tew and Nicholas R Walker Topics in Current Chemistry: Halogen Bonding: Impact on Materials Chemistry and Life Sciences
 Iph accommodation promo
Iph accommodation promo Indice de desenvolvimento humano
Indice de desenvolvimento humano Atomic emission spectra and the quantum mechanical model
Atomic emission spectra and the quantum mechanical model Atomic emmision spectrum
Atomic emmision spectrum Emission and absorption spectra grade 12
Emission and absorption spectra grade 12 Vibrational spectra of metal carbonyls
Vibrational spectra of metal carbonyls Spectra tips
Spectra tips Spectra shropshire
Spectra shropshire Water gas
Water gas Absent spectra in diffraction grating
Absent spectra in diffraction grating Nitro group ir peak
Nitro group ir peak Spectroscopy equations
Spectroscopy equations Azza spectra
Azza spectra Ir spectra of alkanes
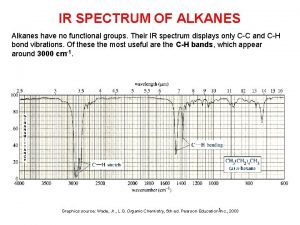
Ir spectra of alkanes