Grade 12 Physics Knowledge area Matter and materials


- Slides: 14

Grade 12 Physics Knowledge area: Matter and materials

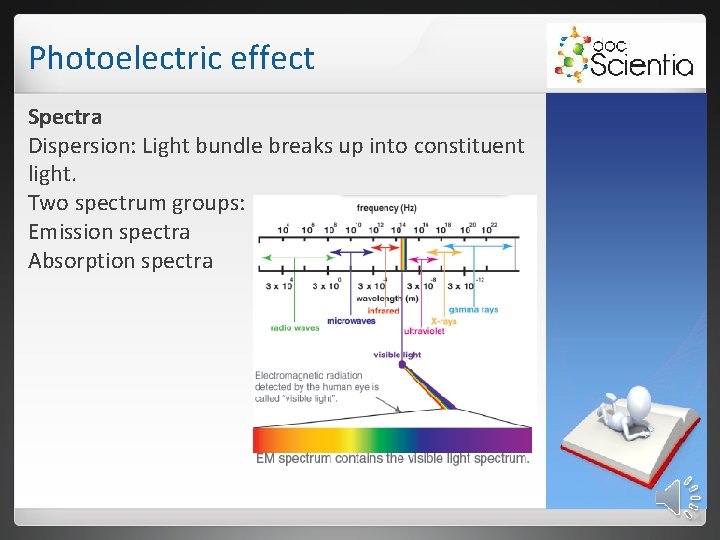
Photoelectric effect Spectra Dispersion: Light bundle breaks up into constituent light. Two spectrum groups: Emission spectra Absorption spectra

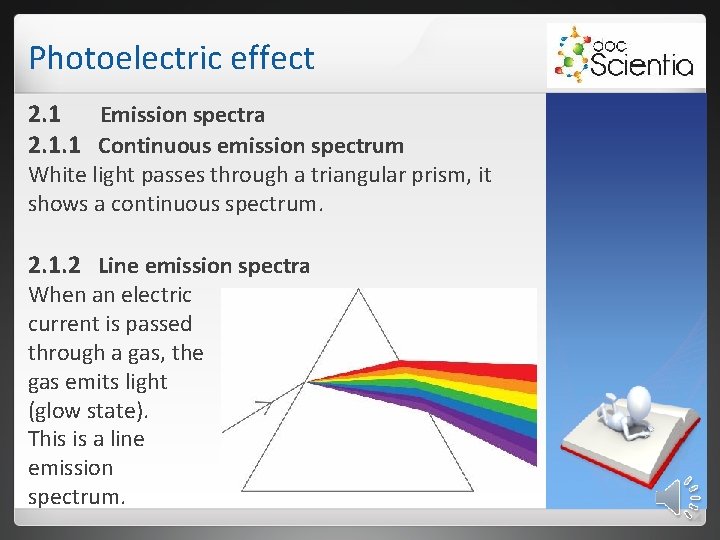
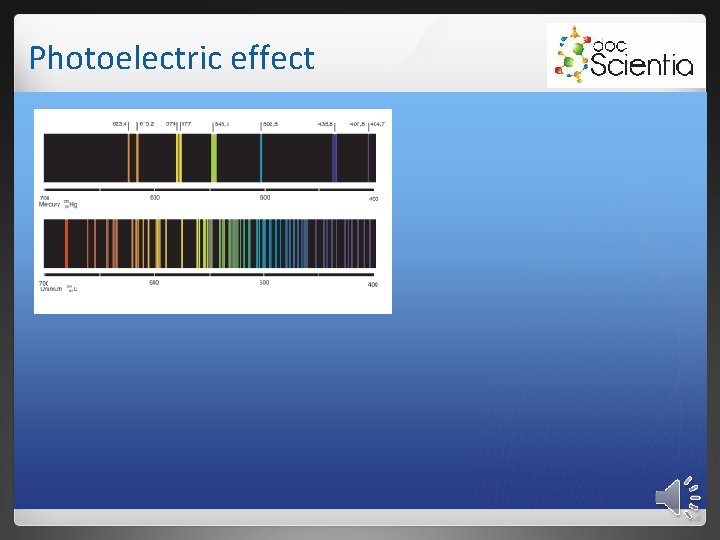
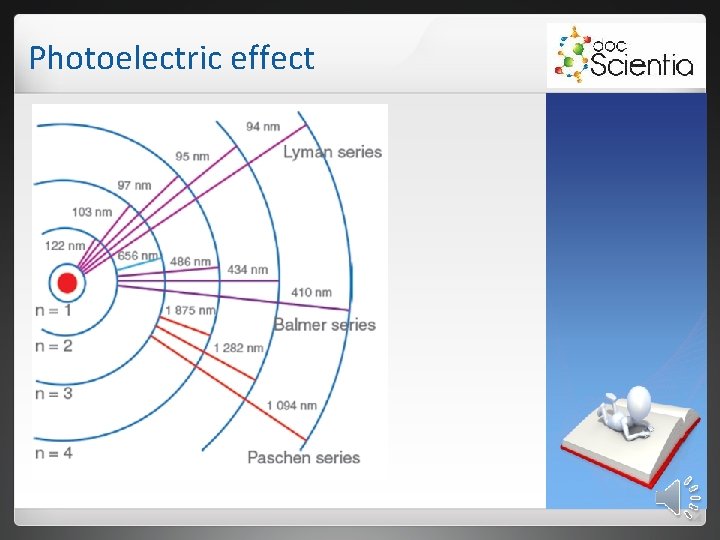
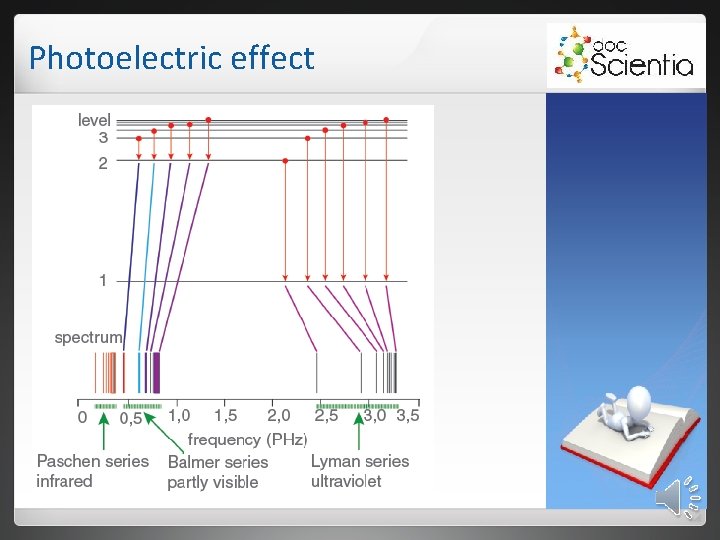
Photoelectric effect 2. 1 Emission spectra 2. 1. 1 Continuous emission spectrum White light passes through a triangular prism, it shows a continuous spectrum. 2. 1. 2 Line emission spectra When an electric current is passed through a gas, the gas emits light (glow state). This is a line emission spectrum.

Photoelectric effect

Photoelectric effect

Photoelectric effect The energy of the photons match the difference in energy between the energy levels into which the electrons fall. Elements have unique energy levels and therefore also unique line emission spectra.

Photoelectric effect

Photoelectric effect

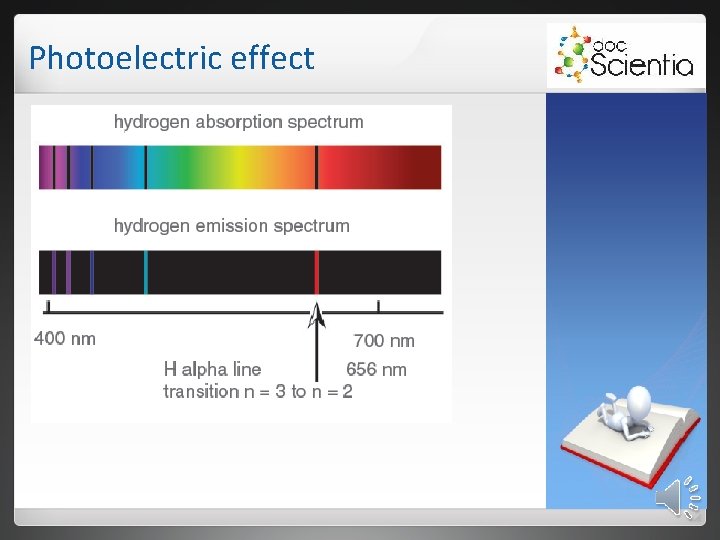
Photoelectric effect 2. 2 Absorption spectra Formed when light is sent through a cold diluted gas. Atoms of the gas absorb certain frequencies. Absorbed frequencies appear as black lines.

Photoelectric effect

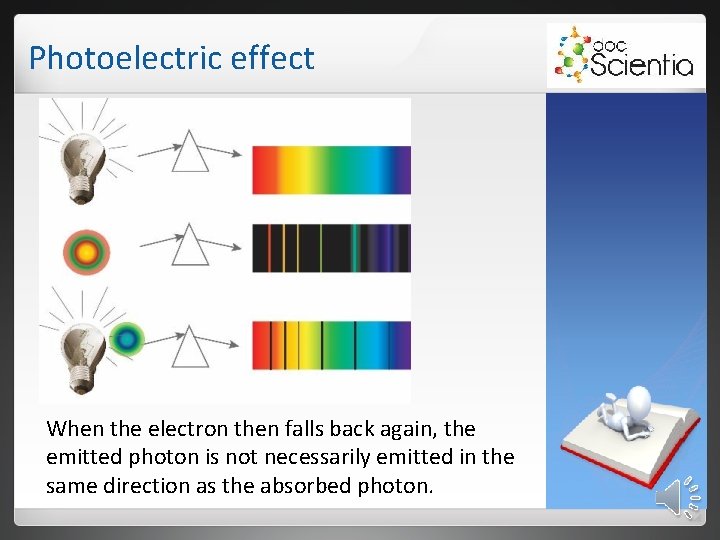
Photoelectric effect When the electron then falls back again, the emitted photon is not necessarily emitted in the same direction as the absorbed photon.

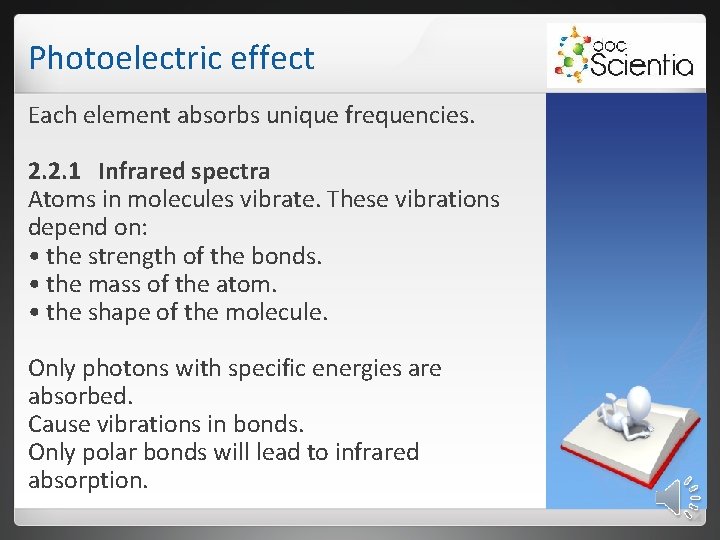
Photoelectric effect Each element absorbs unique frequencies. 2. 2. 1 Infrared spectra Atoms in molecules vibrate. These vibrations depend on: • the strength of the bonds. • the mass of the atom. • the shape of the molecule. Only photons with specific energies are absorbed. Cause vibrations in bonds. Only polar bonds will lead to infrared absorption.

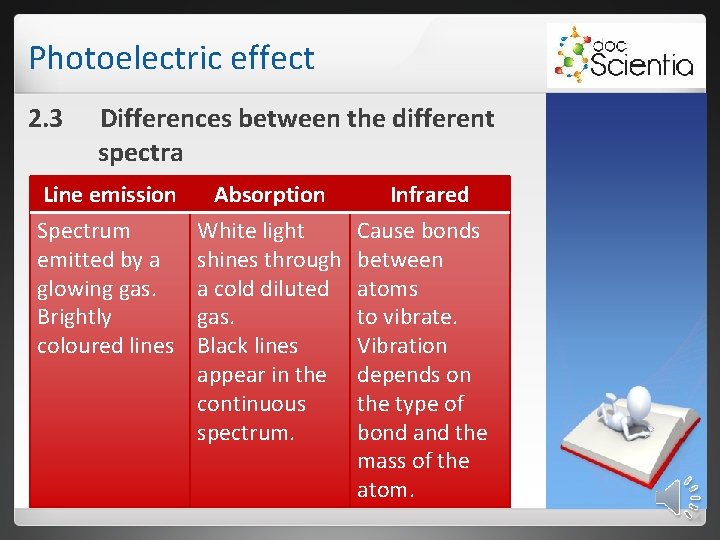
Photoelectric effect 2. 3 Differences between the different spectra Line emission Spectrum emitted by a glowing gas. Brightly coloured lines Absorption White light shines through a cold diluted gas. Black lines appear in the continuous spectrum. Infrared Cause bonds between atoms to vibrate. Vibration depends on the type of bond and the mass of the atom.

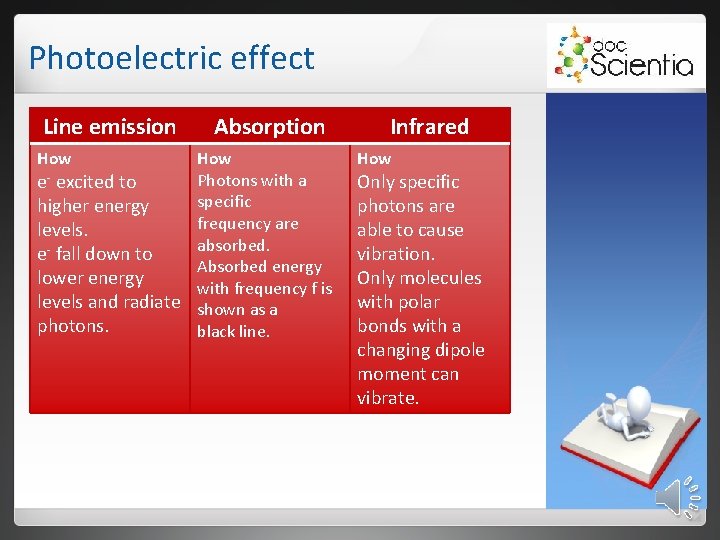
Photoelectric effect Line emission How Absorption How Photons with a e- excited to specific higher energy frequency are levels. absorbed. e- fall down to Absorbed energy lower energy with frequency f is levels and radiate shown as a photons. black line. Infrared How Only specific photons are able to cause vibration. Only molecules with polar bonds with a changing dipole moment can vibrate.