MENOPAUSE MANAGEMENT 2017 UI CARVER COLLEGE OF MEDICINE









![THANK YOU! Menopause Smartset OBG: MENOPAUSE [6294] • Hormone therapies by dose category • THANK YOU! Menopause Smartset OBG: MENOPAUSE [6294] • Hormone therapies by dose category •](https://slidetodoc.com/presentation_image_h/b8434114ef9b23c99bffc63ec94319c2/image-26.jpg)

- Slides: 27

MENOPAUSE MANAGEMENT 2017 UI CARVER COLLEGE OF MEDICINE REFRESHER COURSE FOR THE FAMILY PHYSICIAN, APRIL 18 TH , 11 -11: 30 AM VERONIKA-KOLDER@UIOWA. EDU Disclosures: none

AGENDA § Contraception § Non-hormonal therapy § Hormonal therapy § Stopping hormone therapy http: //littlecunning. Plan. com/2012/10/menopause-sucks/

OBJECTIVES • Provide midlife contraception • Compare efficacy of non-hormonal and hormonal options for vasomotor symptoms • Provide hormone therapy counseling • Stop hormone therapy

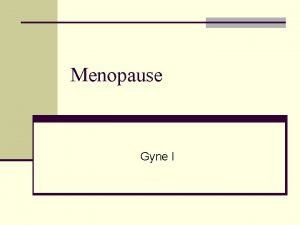
CONTRACEPTION Final Menstrual Period Terminology Menopause Transition Early Postmenopause Late Early Late Perimenopause Duration of stage Menstrual cycle Variable 1 -3 years Variable cycle length (persistent ≥ 7 day difference in length of consecutive cycles) FSH Symptoms ↑Variable 2 years 3 -6 years Remaining lifespan Interval of amenorrhea ≥ 60 None days ↑Variable Vasomotor symptoms likely ↑Variable Vasomotor symptoms most likely ↑Stable Increasing symptoms of urogenital atrophy Stages of Reproductive Aging Workshop (STRAW+10), Menopause 2012; 19(4): 1

CONTRACEPTION: EXPERT ADVISE • FSH >20 IU/L & LH >30 IU/L? • Several months of amenorrhea & FSH ≥ 40 UI/L? . . . Even under these circumstances, flutuations can occur, with a period of ovarian failure followed by resumption of ovarian function. Because variability is the rule, it would be wise to recommend the use of contraeption until the postmenopausal state is definitely established. Stopping OCPs • If low CV risk and slender, continue till age 51, then switch to nonhormonal contraception Changing from OCPs to postmenopausal HT • Consider ‘contraceptive-strength’ HT, then transdermal HT Fritz & Speroff, 8 th ed. , p. 684

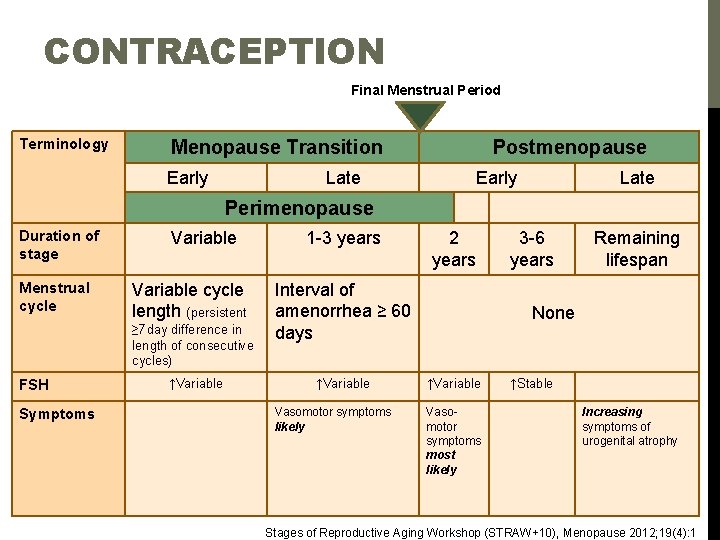
CONTRACEPTIVE COUNSELING Depo Provera Nexplanon Combination OCPs E&P Estrogen Progesterone Levonorgestrel IUD 1 2 3 4 5 6 7 8 …. . . 12 13 14. . . . 26 27 28 Day of Menstrual Cycle v

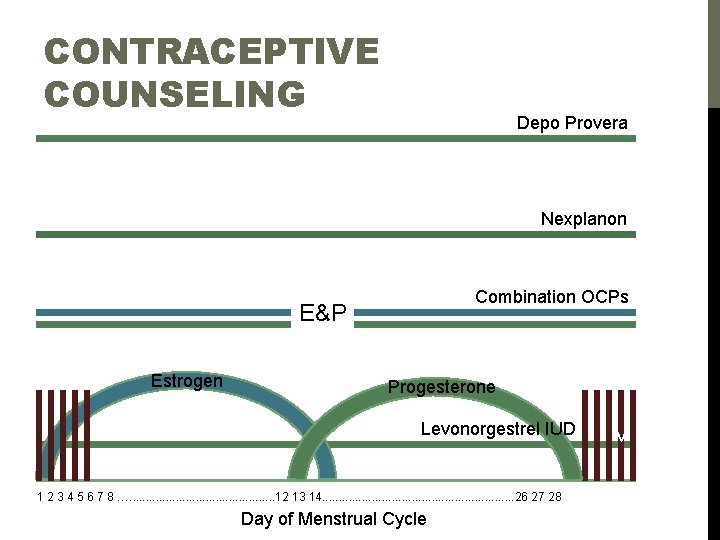
LOW DOSE OCPS & ‘CONTRACEPTIVE-STRENGTH’ HT ***Avoid in obese peri-menopausal females and others at increased VTE risk*** PRODUCT ESTROGEN PROGESTIN Low dose oral contraceptive pills Loestrin® Ethinyl estradiol 20 mcg Norethindrone acetate 1. 0 mg Lo-Loestrin® Ethinyl estradiol 10 mcg Norethindrone acetate 1. 0 mg Lybrel® Ethinyl estradiol 20 mcg Levonorgestrel 90 mcg Mircette® Ethinlx estradiol 20 mcg x 21 d, 2 d inert pills, 10 mcg x 5 d Desogestrel 0. 15 mg x 21 d Yaz® Ethinyl estradiol 20 mcg Drospirenone 3 mg ‘Contaceptive-strength’ oral HT femhrt® Ethinyl estradiol 5 mcg Norethindrone acetate 1. 0 mg** femhrt® (low dose) Ethinyl estradiol 2. 5 mcg Norethindrone acetate 0. 5 mg** Angeliq® Estradiol 1. 0 mg Drospirenone 0. 5 mg** Angeliq® (low dose) Estradiol 0. 5 mg Drospirenone 0. 25 mg* Activella® Estradiol 1. 0 mg Norethindrone acetate 0. 5 mg*** Activella® (low dose) Estradiol 0. 5 mg Norethindrone acetate 0. 1 mg** Prefest® (not recommended by VK) Estradiol 1 mg Norgestimate 0. 09 mg*** *FDA-approved for hot flashes *FDA-approved for vulvo-vaginal atrophy *FDA-approved for prevention osteoporosis

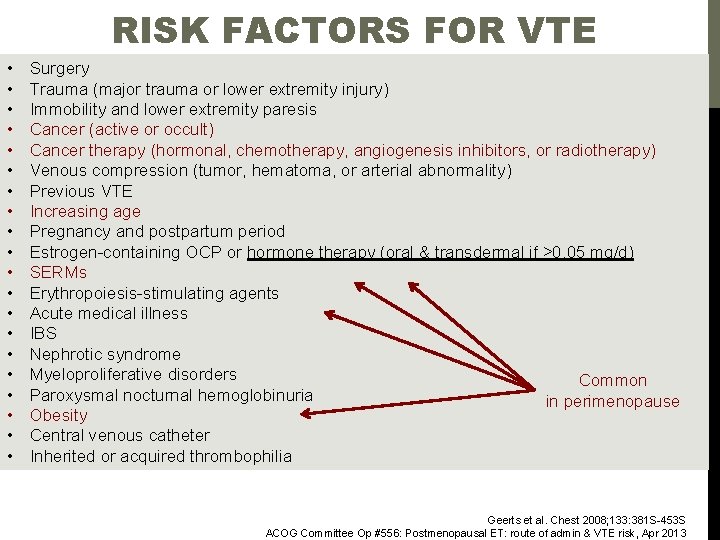
RISK FACTORS FOR VTE • • • • • Surgery Trauma (major trauma or lower extremity injury) Immobility and lower extremity paresis Cancer (active or occult) Cancer therapy (hormonal, chemotherapy, angiogenesis inhibitors, or radiotherapy) Venous compression (tumor, hematoma, or arterial abnormality) Previous VTE Increasing age Pregnancy and postpartum period Estrogen-containing OCP or hormone therapy (oral & transdermal if >0. 05 mg/d) SERMs Erythropoiesis-stimulating agents Acute medical illness IBS Nephrotic syndrome Myeloproliferative disorders Common Paroxysmal nocturnal hemoglobinuria in perimenopause Obesity Central venous catheter Inherited or acquired thrombophilia Geerts et al. Chest 2008; 133: 381 S-453 S ACOG Committee Op #556: Postmenopausal ET: route of admin & VTE risk, Apr 2013

CDC U. S. Medical Eligibility Criteria for Contraceptive Use • Summary chart at: www. cdc. gov/reproductivehealth/unintendedpregnacy/pdf/legal_summarychart_english_final_tag 508. pdf • Migraine with aura • Avoid estrogen due to increased stroke risk • Neurofibromatosis • Avoid progesterone due to risk of tumor growth

PATIENT CENTERED BEST PRACTICES • Adequate time • Detailed history • Limited lab evaluation • Shared decision-making 10 • Return every 2 -3 months

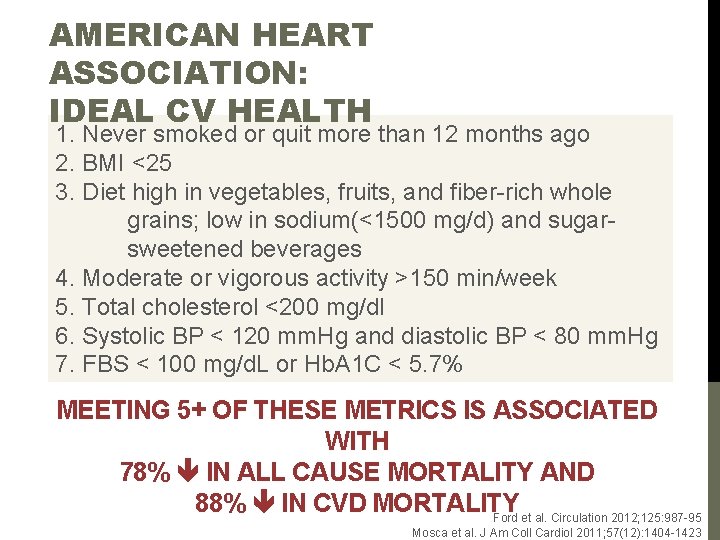
AMERICAN HEART ASSOCIATION: IDEAL CV HEALTH 1. Never smoked or quit more than 12 months ago 2. BMI <25 3. Diet high in vegetables, fruits, and fiber-rich whole grains; low in sodium(<1500 mg/d) and sugarsweetened beverages 4. Moderate or vigorous activity >150 min/week 5. Total cholesterol <200 mg/dl 6. Systolic BP < 120 mm. Hg and diastolic BP < 80 mm. Hg 7. FBS < 100 mg/d. L or Hb. A 1 C < 5. 7% MEETING 5+ OF THESE METRICS IS ASSOCIATED WITH 78% IN ALL CAUSE MORTALITY AND 88% IN CVD MORTALITY Ford et al. Circulation 2012; 125: 987 -95 Mosca et al. J Am Coll Cardiol 2011; 57(12): 1404 -1423

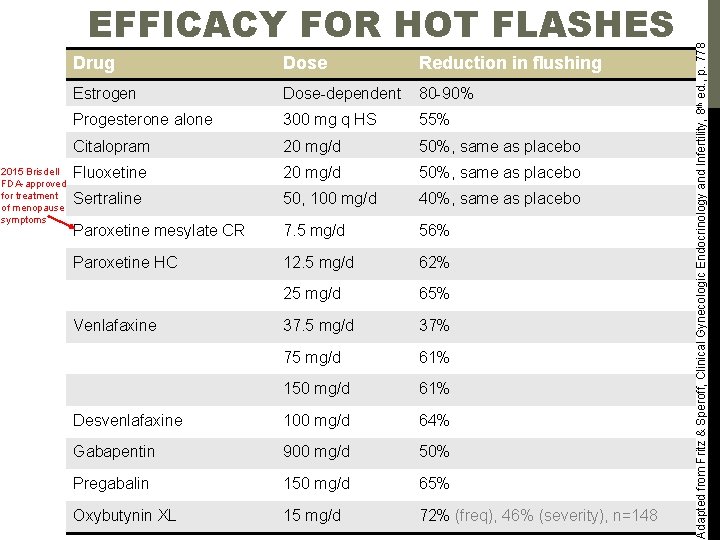
2015 Brisdell FDA-approved for treatment of menopause symptoms Drug Dose Reduction in flushing Estrogen Dose-dependent 80 -90% Progesterone alone 300 mg q HS 55% Citalopram 20 mg/d 50%, same as placebo Fluoxetine 20 mg/d 50%, same as placebo Sertraline 50, 100 mg/d 40%, same as placebo Paroxetine mesylate CR 7. 5 mg/d 56% Paroxetine HC 12. 5 mg/d 62% 25 mg/d 65% 37. 5 mg/d 37% 75 mg/d 61% 150 mg/d 61% Desvenlafaxine 100 mg/d 64% Gabapentin 900 mg/d 50% Pregabalin 150 mg/d 65% Oxybutynin XL 15 mg/d 72% (freq), 46% (severity), n=148 Venlafaxine Adapted from Fritz & Speroff, Clinical Gynecologic Endocrinology and Infertility, 8 th ed. , p. 778 EFFICACY FOR HOT FLASHES

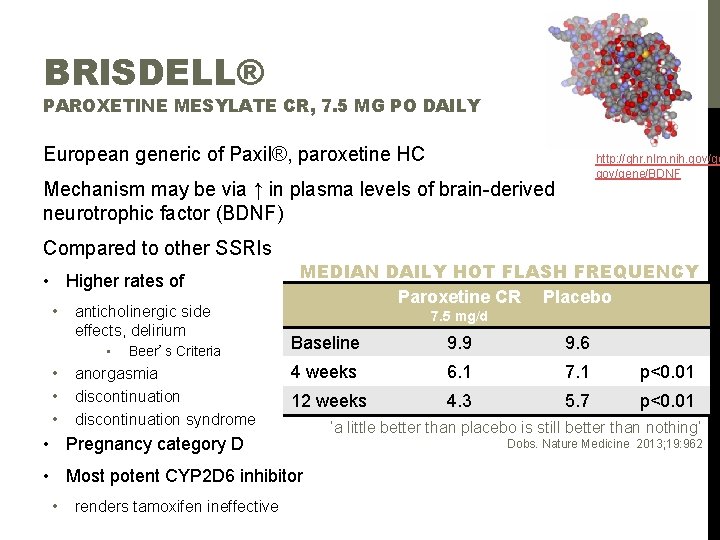
BRISDELL® PAROXETINE MESYLATE CR, 7. 5 MG PO DAILY European generic of Paxil®, paroxetine HC http: //ghr. nlm. nih. gov/gene/BDNF Mechanism may be via ↑ in plasma levels of brain-derived neurotrophic factor (BDNF) Compared to other SSRIs • Higher rates of • anticholinergic side 7. 5 mg/d effects, delirium • • MEDIAN DAILY HOT FLASH FREQUENCY Paroxetine CR Placebo Beer’s Criteria anorgasmia discontinuation syndrome Baseline 9. 9 9. 6 4 weeks 6. 1 7. 1 p<0. 01 12 weeks 4. 3 5. 7 p<0. 01 • Pregnancy category D • Most potent CYP 2 D 6 inhibitor • renders tamoxifen ineffective ‘a little better than placebo is still better than nothing’ Dobs. Nature Medicine 2013; 19: 962

FREE APP Includes: • Contraindications • List of lifestyle modifications • Mentions non-hormonal options • When to choose transdermal over oral • Links to • • Breast Cancer Risk Assessment Tool FRAX calculator www. menopause. org Integrates WHI age-based subgroup analysis with ACC/AHA 10 -year CVD risk prediction score

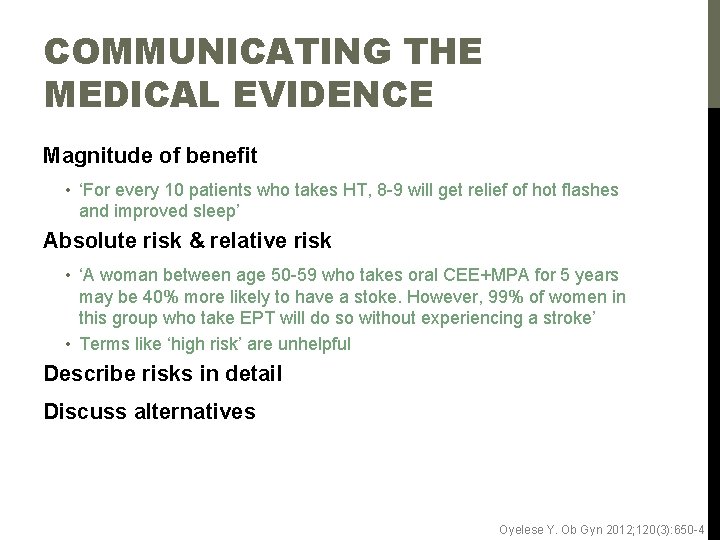
COMMUNICATING THE MEDICAL EVIDENCE Magnitude of benefit • ‘For every 10 patients who takes HT, 8 -9 will get relief of hot flashes and improved sleep’ Absolute risk & relative risk • ‘A woman between age 50 -59 who takes oral CEE+MPA for 5 years may be 40% more likely to have a stoke. However, 99% of women in this group who take EPT will do so without experiencing a stroke’ • Terms like ‘high risk’ are unhelpful Describe risks in detail Discuss alternatives Oyelese Y. Ob Gyn 2012; 120(3): 650 -4

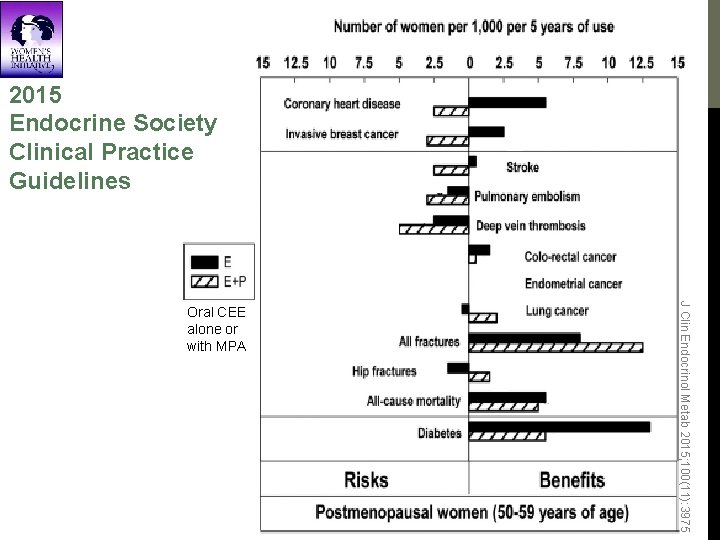
2015 Endocrine Society Clinical Practice Guidelines J Clin Endocrinol Metab 2015; 100(11): 3975 Oral CEE alone or with MPA

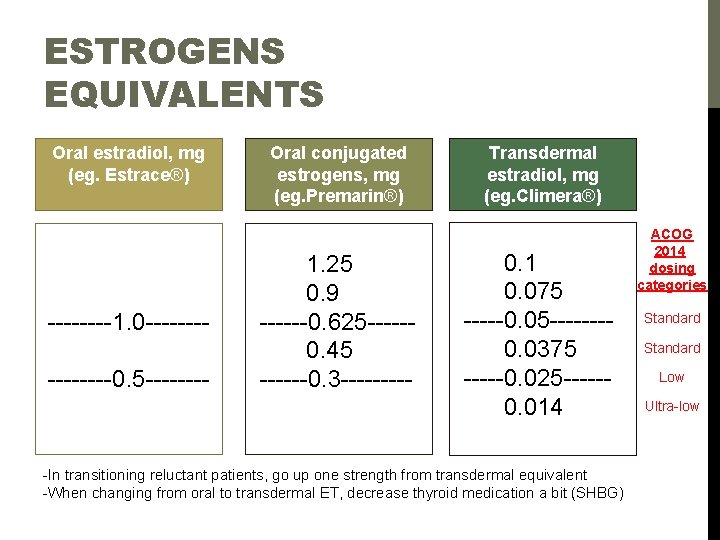
ESTROGENS EQUIVALENTS Oral estradiol, mg (eg. Estrace®) ----1. 0 --------0. 5 ---- Oral conjugated estrogens, mg (eg. Premarin®) 1. 25 0. 9 ------0. 625 ----- 0. 45 ------0. 3 ----- Transdermal estradiol, mg (eg. Climera®) ACOG 2014 dosing categories 0. 1 0. 075 Standard -----0. 05 ---- Standard 0. 0375 Low -----0. 025 -----Ultra-low 0. 014 -In transitioning reluctant patients, go up one strength from transdermal equivalent -When changing from oral to transdermal ET, decrease thyroid medication a bit (SHBG)

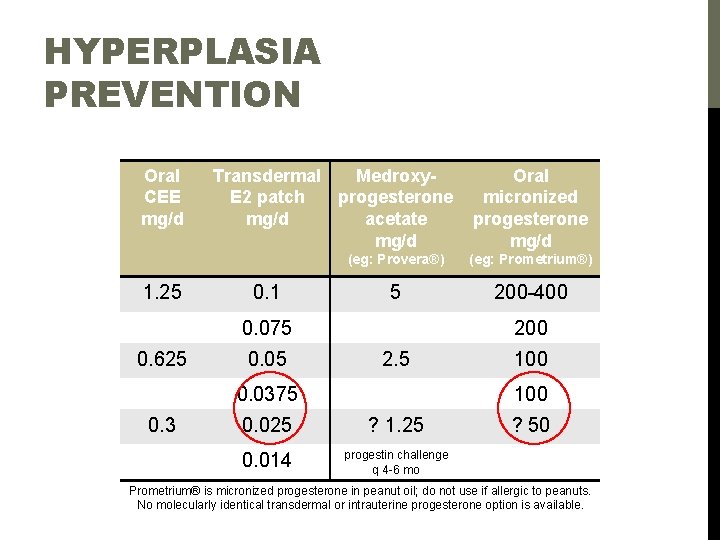
HYPERPLASIA PREVENTION Oral CEE mg/d 1. 25 Transdermal Medroxy. E 2 patch progesterone mg/d acetate mg/d 0. 1 (eg: Provera®) (eg: Prometrium®) 5 200 -400 0. 075 0. 625 0. 05 200 2. 5 0. 0375 0. 3 Oral micronized progesterone mg/d 100 0. 025 ? 1. 25 0. 014 progestin challenge q 4 -6 mo ? 50 Prometrium® is micronized progesterone in peanut oil; do not use if allergic to peanuts. No molecularly identical transdermal or intrauterine progesterone option is available.

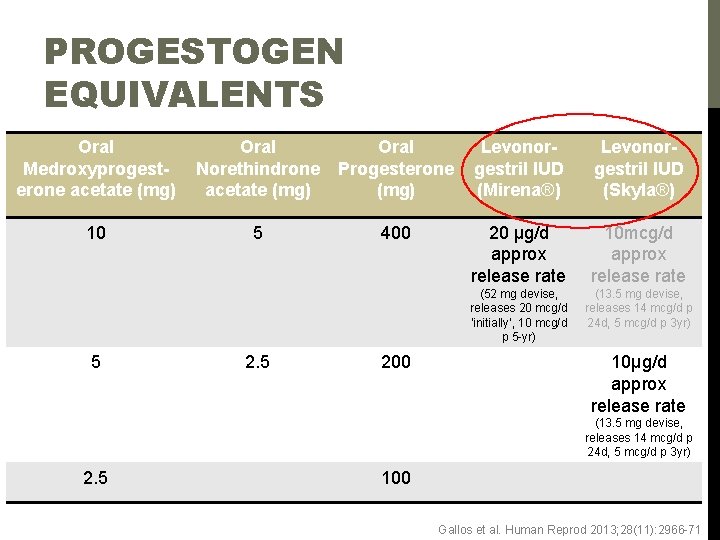
PROGESTOGEN EQUIVALENTS Oral Medroxyprogesterone acetate (mg) Oral Norethindrone acetate (mg) Oral Progesterone (mg) Levonorgestril IUD (Mirena®) Levonorgestril IUD (Skyla®) 10 5 400 20 μg/d approx release rate 10 mcg/d approx release rate (52 mg devise, releases 20 mcg/d ‘initially’, 10 mcg/d p 5 -yr) (13. 5 mg devise, releases 14 mcg/d p 24 d, 5 mcg/d p 3 yr) 5 200 10μg/d approx release rate (13. 5 mg devise, releases 14 mcg/d p 24 d, 5 mcg/d p 3 yr) 2. 5 100 Gallos et al. Human Reprod 2013; 28(11): 2966 -71

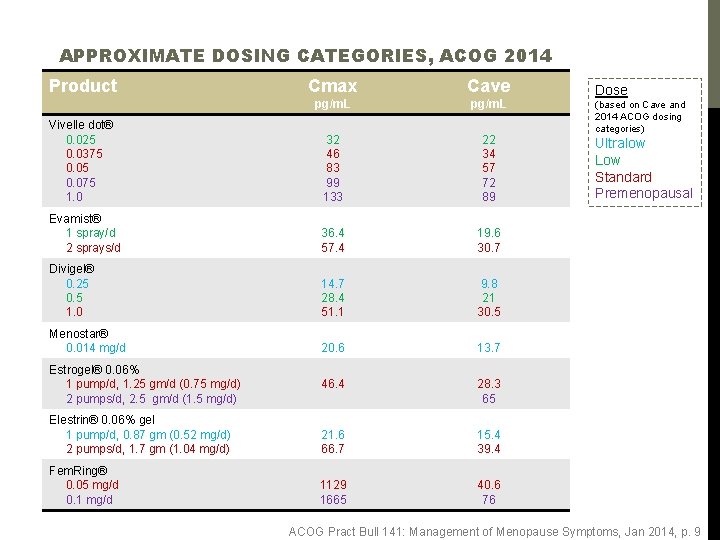
APPROXIMATE DOSING CATEGORIES, ACOG 2014 Product Cmax Cave pg/m. L Vivelle dot® 0. 025 0. 0375 0. 075 1. 0 32 46 83 99 133 22 34 57 72 89 Evamist® 1 spray/d 2 sprays/d 36. 4 57. 4 19. 6 30. 7 Divigel® 0. 25 0. 5 1. 0 14. 7 28. 4 51. 1 9. 8 21 30. 5 Menostar® 0. 014 mg/d 20. 6 13. 7 46. 4 28. 3 65 Elestrin® 0. 06% gel 1 pump/d, 0. 87 gm (0. 52 mg/d) 2 pumps/d, 1. 7 gm (1. 04 mg/d) 21. 6 66. 7 15. 4 39. 4 Fem. Ring® 0. 05 mg/d 0. 1 mg/d 1129 1665 40. 6 76 Estrogel® 0. 06% 1 pump/d, 1. 25 gm/d (0. 75 mg/d) 2 pumps/d, 2. 5 gm/d (1. 5 mg/d) Dose (based on Cave and 2014 ACOG dosing categories) Ultralow Low Standard Premenopausal ACOG Pract Bull 141: Management of Menopause Symptoms, Jan 2014, p. 9

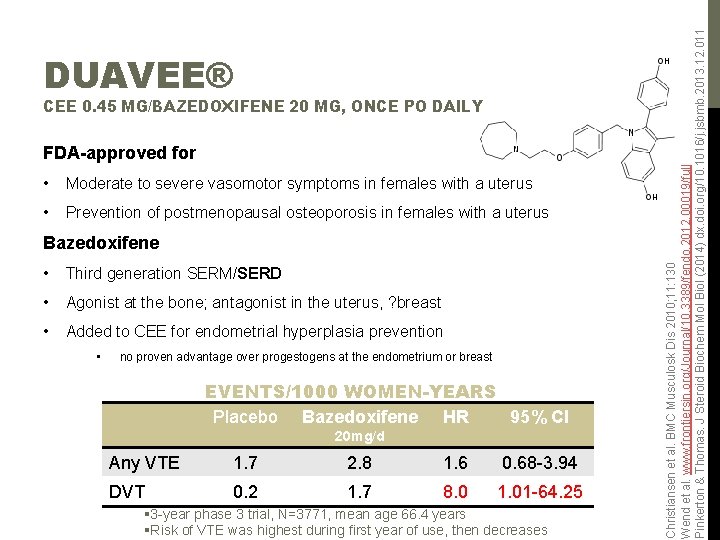
CEE 0. 45 MG/BAZEDOXIFENE 20 MG, ONCE PO DAILY FDA-approved for • Moderate to severe vasomotor symptoms in females with a uterus • Prevention of postmenopausal osteoporosis in females with a uterus Bazedoxifene • Third generation SERM/SERD • Agonist at the bone; antagonist in the uterus, ? breast • Added to CEE for endometrial hyperplasia prevention • no proven advantage over progestogens at the endometrium or breast EVENTS/1000 WOMEN-YEARS Placebo Bazedoxifene HR 95% CI 20 mg/d Any VTE 1. 7 2. 8 1. 6 0. 68 -3. 94 DVT 0. 2 1. 7 8. 0 1. 01 -64. 25 § 3 -year phase 3 trial, N=3771, mean age 66. 4 years §Risk of VTE was highest during first year of use, then decreases Christiansen et al. BMC Musculosk Dis 2010; 11: 130 Wend et al. www. frontiersin. org/Journal/10. 3389/fendo. 2012. 00019/full Pinkerton & Thomas. J Steroid Biochem Mol Biol (2014) dx. doi. org/10. 1016/j. jsbmb. 2013. 12. 011 DUAVEE®

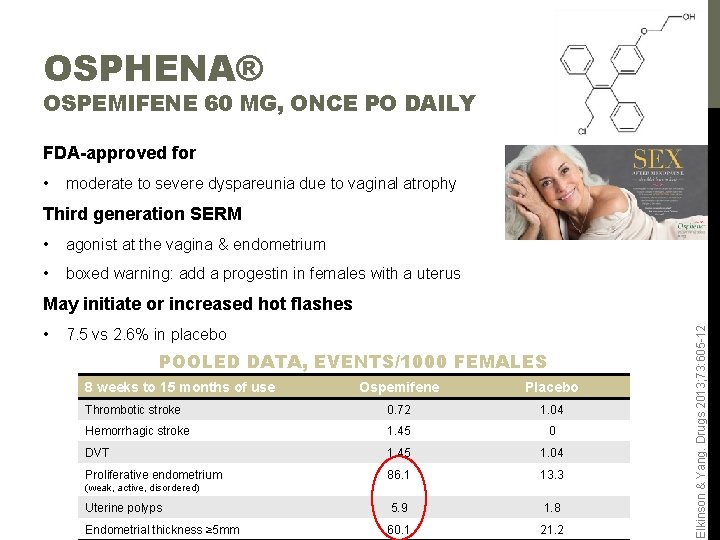
OSPHENA® OSPEMIFENE 60 MG, ONCE PO DAILY FDA-approved for • moderate to severe dyspareunia due to vaginal atrophy Third generation SERM • agonist at the vagina & endometrium • boxed warning: add a progestin in females with a uterus • 7. 5 vs 2. 6% in placebo POOLED DATA, EVENTS/1000 FEMALES 8 weeks to 15 months of use Ospemifene Placebo Thrombotic stroke 0. 72 1. 04 Hemorrhagic stroke 1. 45 0 DVT 1. 45 1. 04 Proliferative endometrium 86. 1 13. 3 Uterine polyps 5. 9 1. 8 Endometrial thickness ≥ 5 mm 60. 1 21. 2 (weak, active, disordered) Elkinson & Yang. Drugs 2013; 73: 605 -12 May initiate or increased hot flashes

STOPPING HT • RCTs suggests gradual reduction and abrupt cessation equally likely to result in recurrent symptoms • 15% of females have bothersome hot flashes into their 80’s • If on oral HT, switch to transdermal HT, then gradually reduce dose • The breast safety profile of hysterectomized females who are on estrogen alone allows consideration of more long term use • stopping or continuing HT involves shared decision-making which should be documented • In older females or those with CVD risk factors, consider stopping (or restarting) HT gradually to minimize rapid vasodilating effects of estradiol Fritz & Speroff, Clinical Gynecologic Endocrinology and Infertility, 8 th ed

ACCESS TO HORMONE THERAPY Beers Criteria for Potentially Inappropriate Medication Use in Older Adults Oral and patch systemic HT: • strong recommendation to avoid based on high quality evidence Topical vaginal cream or tablets: • weak recommendation to avoid based on moderate quality evidence Have been challenged by the American College of Ob Gyn and the North American Menopause Society • Sample insurance letters available on request • Urogyn division prescribes compounded vaginal estriol cream AGS. J Am Geriatr Soc 2015; 2227 -46 NAMS. Menopause 2015; 22: 693

TAKE HOME MESSAGES Continue contraception until one year after the final menstrual period Non-hormonal treatment options for hot flashes are less effective Just because the WHI was the mother of all RCTs doesn’t mean her results have external validity Avoid new SERMS when alternatives with less VTE risk are available The breast safety profile for hysterectomized females taking estrogen alone allows consideration of longer term use Continuing postmenopausal hormone therapy should be an individualized, shared decision
![THANK YOU Menopause Smartset OBG MENOPAUSE 6294 Hormone therapies by dose category THANK YOU! Menopause Smartset OBG: MENOPAUSE [6294] • Hormone therapies by dose category •](https://slidetodoc.com/presentation_image_h/b8434114ef9b23c99bffc63ec94319c2/image-26.jpg)
THANK YOU! Menopause Smartset OBG: MENOPAUSE [6294] • Hormone therapies by dose category • • • Standard Low Ultralow Other menopause providers: • Labs for • • Hair loss Pheochromocytoma Celiac disease Metabolic causes of bone loss • Elizabeth Graf, PA-C, NCMP • Eugenia Mazur, MD, NCMP • Marygrace Elson, MD, NCMP • Rachel Mejia, MD, NCMP: North American Menopause Society (NAMS)-Certified Menopause Practitioner Corrections, questions, & comments welcome at veronika-kolder@uiowa. edu Menopause and Sexual Health Clinic protocols available on request.

RESOURCES (ALPHABETICAL) American College of Obstetrics and Gynecology, Committee Opinion 556, Postmenopausal ET: Route of Administration and Risk of VTE. Obstetr Gynecol 2013; 121(4): 887 -90 North American Menopause Society position statement, Nonhormonal management of menopause-associated vasomotor symptoms. Menopause 2015; 22(11): 1 -18. North American Menopause Society statement on HT use after age 65. Menopause 2015; 22: 693 Stuenkel CA et al. Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2015; 100(11): 39754011
 Symplex f for menopause
Symplex f for menopause Menopause definition
Menopause definition What is menopause
What is menopause Premature menopause quiz
Premature menopause quiz Menopause wann
Menopause wann Dr wilson adrenal rebuilder side effects
Dr wilson adrenal rebuilder side effects Menopas
Menopas Will you please be quiet please raymond carver
Will you please be quiet please raymond carver Gw carver middle school miami
Gw carver middle school miami Gw carver middle school uniforms
Gw carver middle school uniforms Matt carver sai
Matt carver sai Restorative dental instruments
Restorative dental instruments William veasey decoy carver
William veasey decoy carver Raymond carver alcoholism
Raymond carver alcoholism Carver dress code
Carver dress code Site:slidetodoc.com
Site:slidetodoc.com First day of each season
First day of each season Come orientarsi con le stelle
Come orientarsi con le stelle Ash 49 dental instrument
Ash 49 dental instrument Kris carver
Kris carver Pamela carver
Pamela carver Pam carver
Pam carver Penn state college of medicine msr
Penn state college of medicine msr 纽约中医学院
纽约中医学院 Lincoln memorial university college of veterinary medicine
Lincoln memorial university college of veterinary medicine King faisal university college of medicine
King faisal university college of medicine King saud university college of medicine
King saud university college of medicine Prodofol
Prodofol