Medical Marijuana Denis J Petro MD Cynosure Neuroscience



























- Slides: 30

Medical Marijuana Denis J. Petro, MD Cynosure Neuroscience



ANA Position Statement on Medical Marijuana 1. The education of registered nurses and other health care practitioners regarding appropriate evidence-based therapeutic use of marijuana including those nonsmoked forms of delta-9 -tetrahydrocannabinol (THC) that have proven to be therapeutically efficacious 2. Protection from criminal or civil penalties for patients using medical marijuana as permitted under state laws 3. Exemption from criminal prosecution; civil liability; or professional sanctioning, such as loss of licensure or credentialing, for health care practitioners who prescribe, dispense or administer medical marijuana in accordance with state law. 4. Reclassification of marijuana’s status from a Schedule I controlled substance into a less restrictive category. 5. Confirmation of therapeutic efficacy of medical marijuana.

Context • • • History of Cannabis as Medicine How Science Works Pharmaceutical Industry Regulatory Authorities Criminal Justice System Media



Personal Experience • • • Observations 1960 s TIRR, VA system 1970 s Penn State Univ. FDA 1980 s-00 s Research

Cannabis in Cancer Chemotherapy • Neurology. 1978 Feb; 28(2): 174 -8. Neurologic complications of acute myelomonoblastic leukemia of four years' duration. ・Ballard JO, Towfighi J, Brennan RW, Saleem S, Eyster ME. An adult with acute nonlymphoblastic leukemia involving the central nervous system is presented. Unusual features included: (1) Focal signs and radiographic evidence of sagittal sinus occlusion early in the course of disease; (2) progressive meningeal, cranial nerve, and spinal nerve involvement despite a 4 -year bone marrow remission; (3) intracerebral tumor formation, and (4) retrobulbar optic neuritis associated with microscopic findings of herpeslike viral particles. The incidence of clinically overt neurologic disease in adults with acute nonlymphoblastic leukemia seems to have increased in tandem with improved chemotherapy. The prophylactic treatment of the central nervous system during prolonged remission of adult acute nonlymphoblastic leukemia may prove of benefit to these patients. PMID: 271773 [Pub. Med - indexed for MEDLINE]

Petro DJ. Marihuana as a therapeutic agent for muscle spasm or spasticity. Psychosomatics 1980 21: 81 - 85. This is a case report of two cases, one of whom had MS. Nocturnal leg spasms were relieved by smoking cannabis within five minutes. Abstention led to increased spasticity and pain, again relieved by use of cannabis.

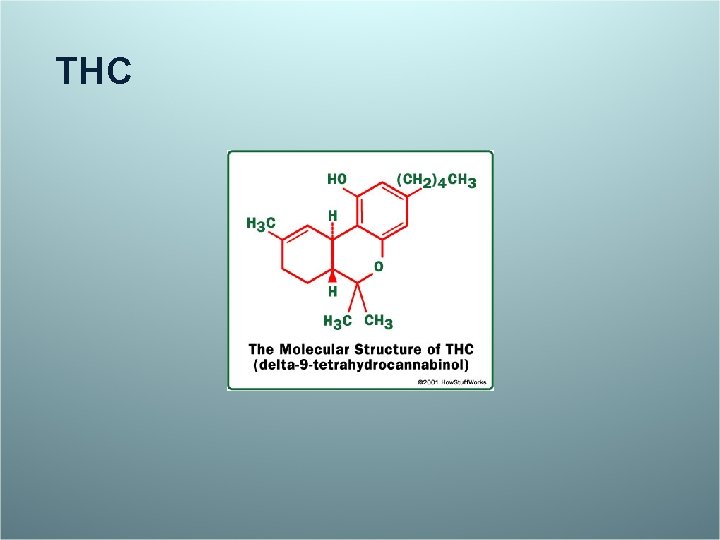
THC

Petro & Ellenberger. Treatment of human spasticity with delta 9 -tetrahydrocannabinol . J Clin Pharmacol 1981 21: (8 -9 Suppl): 413 S -416 S. Nine patients with spasticity related to MS were examined by a blinded observer before and after 90 minutes intervals after oral capsules with 10 mg g, 5 mg or no synthetic THC, but not placebo, was associated with a reduced spasticity score lasting for about 4 hours. Big improvements with 4/9 with THC and 1/9 with placebo. Subjective highs were experienced by one patient after THC and one after placebo.

Joseph Heller Catch 22 • There was only one catch and that was Catch-22, which specified that a concern for one's safety in the face of dangers that were real and immediate was the process of a rational mind. Orr was crazy and could be grounded. All he had to do was ask; and as soon as he did, he would no longer be crazy and would have to fly more missions. Orr would be crazy to fly more missions and sane if he didn't, but if he was sane he had to fly them. If he flew them he was crazy and didn't have to; but if he didn't want to he was sane and had to. Yossarian was moved very deeply by the absolute simplicity of this clause of Catch-22 and let out a respectful whistle. "That's some catch, that Catch-22, " he [Yossarian] observed. "It's the best there is, " Doc Daneeka agreed.

U. S. Government Compassionate Investigational New Drug Program Federal program created to fit within scope of the 1961 UN Single Convention on Narcotic Drugs

Malec et al. Cannabis effect on spasticity in spinal cord injury. Arch Phys Med rehabil 1982 63: 116 -8. Questionnaire to spinal cord injury patients. 9/24 users reported no spasticity while using cannabis, 11/24 reported some benefit.

Marinol 1986 Delta-9 THC in capsule formulation Approved for Nausea & Vomiting due to Cancer Chemo Appetite/weight loss due to AIDS Oral use-in sesame oil (10 -20% absorbed) Onset in 1/2 to 1 hr, Peak 2 -4 hr, 1/2 life 4 hr Single dose with detectable metabolites > 35 days

DEA Petition 1986 -88 Request to move Marijuana to DEA Schedule II filed in 1972 -Hearings lasted from 1986 to 1988 -Affidavit filed by DJP, called to testify by DEA -Judge Young ruled that Marijuana should be reclassified as a Schedule II drug under CSA and made available for medical purposes (September 6, 1988) -DEA ignored ruling

Meinck et al. Effect of cannabinoids on spasticity and ataxia in multiple sclerosis. J Neurol 1989 263: 120 -2. Chronic motor handicaps of one MS patient improved acutely while smoking cannabis cigarette.

Cannabinoid Drugs 1986 • • • delta-9 -Tetrahydrocannabinol (Δ 9 -THC, THC) and delta-8 -tetrahydrocannabinol (Δ 8 THC), mimic the action of anandamide. The THCs produce the high associated with cannabis by binding to the CB 1 cannabinoid receptors in the brain. Cannabidiol (CBD), non-psychoactive. CBD has anti-inflammatory effects. CBD shares a precursor with THC and is the main cannabinoid in low-THC Cannabis strains. Cannabinol (CBN), a degradation product of THC, produces a depressant effect.

Greenberg et al. Short-term effects of smoking marijuana on balance in patients with multiple sclerosis and normal volunteers. Clin Pharm Ther 1994 55: 324 -8. Randomised, double blind study of inhaled cannabis on postural (balance) responses in normal subjects and MS patients. Technical paper that says that cannabis gives patients and MS sufferers worse balance control.

1990 s Cannabinoid receptors USA State Propositions Prosecutions IOM report

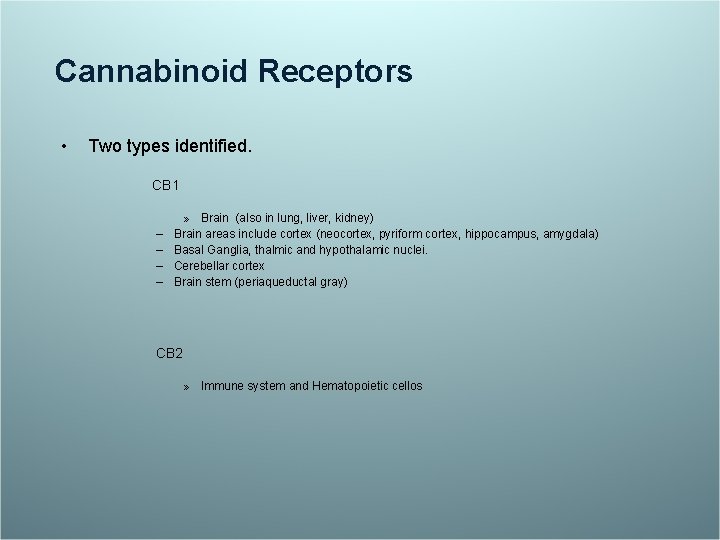
Cannabinoid Receptors • Two types identified. CB 1 – – » Brain (also in lung, liver, kidney) Brain areas include cortex (neocortex, pyriform cortex, hippocampus, amygdala) Basal Ganglia, thalmic and hypothalamic nuclei. Cerebellar cortex Brain stem (periaqueductal gray) CB 2 » Immune system and Hematopoietic cellos



IOM Report 1998 White House Office of National Drug Control Policy (ONDCP) requested a review of the scientific evidence re: health benefits and risks of Marijuana and constituent cannabinoids. Response to 1996 referenda in California and Arizona permitting the use of Marijuana as medicine. Three 2 -day workshops (Irvine, New Orleans, Washington, DC) gathering scientific input.

IOM Recommendations • • • 1. Research should continue into effects of synthetic and plant-derived cannabinoids. Different cannabinoids have different effects. Focus on pain relief, control of nausea and vomiting, and appetite stimulation. 2. Clinical trials should be conducted to develop rapid-onset, reliable, and safe delivery systems. 3. Psychological effects such as anxiety reduction and sedation should be evaluated in clinical trials. 4. Studies to define the individual health risks of smoking marijuana should be conducted, particularly among populations in which marijuana use is prevalent. Smoked marijuana is a crude delivery system and not recommended for medical use. 5. Clinical trials should focus on short term use (less than 6 months) in conditions for which there is a reasonable expectation of efficacy with IRB approval and with data relative to efficacy. Isolated cannabinoids and their synthetic derivatives offer the best choices for clinical study and clinical trials of smoked marijuana should serve as a first step toward the development of nonsmoked, rapid-onset cannabinoid delivery systems. 6. Short-term use of smoked marijuana (less than 6 months) for patients with debilitating symptoms must meet the following conditions: – – Failure of all approved medications to provide relief The symptoms can reasonably be expected to be relieved by rapid-onset cannabinoids Treatment is administered under medical supervision with efficacy assessment; and IRB guidance and oversight.

Mechanism of Action in Spasticity • Nature 404, 84 -87 (2 March 2000) | doi: 10. 1038/35003583; Received 18 August 1999; Accepted 20 January 2000 • Cannabinoids control spasticity and tremor in a multiple sclerosis model • David Baker 1, Gareth Pryce 1, J. Ludovic Croxford 1, Peter Brown 2, Roger G. Pertwee 3, John W. Huffman 4 and Lorna Layward 1. Neuroinflammation Group, Department of Neurochemistry, Institute of Neurology, University College London, 1 Wakefield Street, London WC 1 N 1 PJ and the Institute of Ophthalmology, UCL, London EC 1 V 9 EL, UK 2. The Medical Research Council Human Movement and Balance Unit, National Hospital for Neurology and Neurosurgery , Queen Square, London, WC 1 N 3 BG, UK 3. Department of Biomedical Sciences, Institute of Medical Sciences, University of Aberdeen, Foresterhill , Aberdeen AB 25 2 ZD, UK 4. Department of Chemistry, Clemson University, Clemson, South Carolina 29634 -1905 , USA 5. Multiple Sclerosis Society of Great Britain and Northern Ireland , 25 Effie Road, London SW 6

Mechanism (continued) • • Chronic relapsing experimental allergic encephalomyelitis (CREAE) is an autoimmune model of multiple sclerosis. Although both these diseases are typified by relapsing-remitting paralytic episodes, after CREAE induction by sensitization to myelin antigens Biozzi ABH mice also develop spasticity and tremor. These symptoms also occur during multiple sclerosis and are difficult to control. This has prompted some patients to find alternative medicines, and to perceive benefit from cannabis use. Although this benefit has been backed up by small clinical studies, mainly with non-quantifiable outcomes, the value of cannabis use in multiple sclerosis remains anecdotal. Here we show that cannabinoid (CB) receptor agonism using R(+)-WIN 55, 212, 9 tetrahydrocannabinol, methanandamide and JWH-133 quantitatively ameliorated both tremor and spasticity in diseased mice. The exacerbation of these signs after antagonism of the CB 1 and CB 2 receptors, notably the CB 1 receptor, using SR 141716 A and SR 144528 indicate that the endogenous cannabinoid system may be tonically active in the control of tremor and spasticity. This provides a rationale for patients' indications of therapeutic potential of cannabis in the control of the symptoms of multiple sclerosis, and provides a means of evaluating more selective cannabinoids in the future.

2000 s State Laws enacted for patient access GW Pharmaceuticals. Sativex

GW Pharmaceutical PLC -UK-based pharmaceutical company developing Sativex Oromucosal Spray. -Available in 26 countries around the world. -Sativex approved by Health Canada in 2005 for the relief of neuropathic pain in MS (marketed by Bayer). -Began clinical trial of Sativex in USA in 2007 as adjunctive treatment of cancer pain.
 Medical marijuana ethics
Medical marijuana ethics Ohio medical marijuana day supply chart
Ohio medical marijuana day supply chart Should marijuana be a medical option
Should marijuana be a medical option Maria petro teacher
Maria petro teacher Petro pariñas sac houston
Petro pariñas sac houston Petro kononova
Petro kononova Sutura petro giugulare
Sutura petro giugulare Petro-tech heat technology inc
Petro-tech heat technology inc Petrooxy
Petrooxy Joanne supervised 36 professionals in 6 city libraries
Joanne supervised 36 professionals in 6 city libraries Opw fuel management systems inc
Opw fuel management systems inc Petrol pump design plan
Petrol pump design plan Became marijuana state
Became marijuana state Lungs marijuana
Lungs marijuana Brown weeds
Brown weeds Marijuana names
Marijuana names Porter neuroscience research center
Porter neuroscience research center Collaborative neuroscience research llc
Collaborative neuroscience research llc Neuroscience major usc
Neuroscience major usc Halle berry
Halle berry Systems neuroscience textbook
Systems neuroscience textbook Australian neuroscience nurses association
Australian neuroscience nurses association Federation of european neuroscience societies
Federation of european neuroscience societies Uw madison neuroscience
Uw madison neuroscience Purves neuroscience
Purves neuroscience Unsolved problems in neuroscience
Unsolved problems in neuroscience Dynamical systems neuroscience
Dynamical systems neuroscience Max planck institute neuroscience internship
Max planck institute neuroscience internship Neuroscience example
Neuroscience example Why is chess addictive
Why is chess addictive Developmental neuroscience textbook
Developmental neuroscience textbook