MAHATMA PHULE A S C COLLEGE PANVEL PHOTOCHEMISTRY


![K 1 Iabs=k 2[B] or K 2/k 1=Iabs/[B] or K=I abs/[B] Where K=K 2/k K 1 Iabs=k 2[B] or K 2/k 1=Iabs/[B] or K=I abs/[B] Where K=K 2/k](https://slidetodoc.com/presentation_image_h2/57de4df3390c3e2f7cb60fefe89751e3/image-15.jpg)







- Slides: 29

MAHATMA PHULE A. S. C COLLEGE, PANVEL PHOTOCHEMISTRY CLASS – S. Y. B. Sc. Paper -1 [Physical and Analytical Chemistry] By – Mr. Katkar R. J. Dept. Of Chemistry

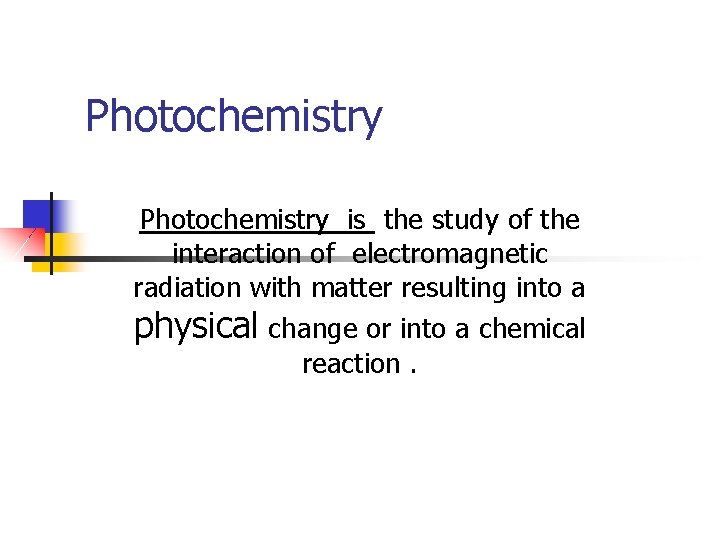
Photochemistry is the study of the interaction of electromagnetic radiation with matter resulting into a physical change or into a chemical reaction.

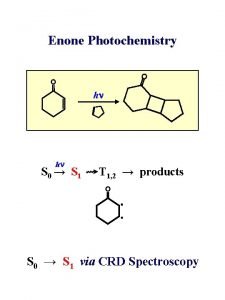
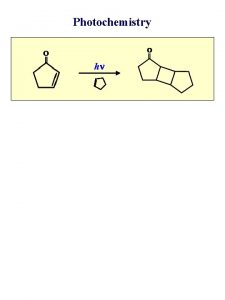
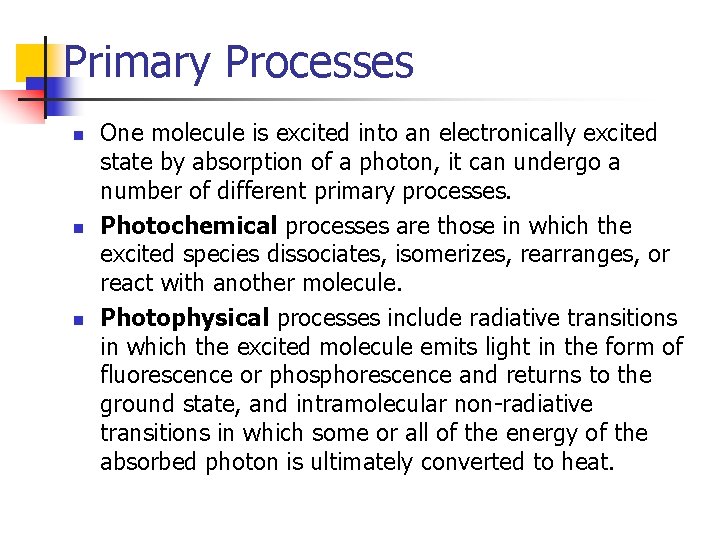
Primary Processes n n n One molecule is excited into an electronically excited state by absorption of a photon, it can undergo a number of different primary processes. Photochemical processes are those in which the excited species dissociates, isomerizes, rearranges, or react with another molecule. Photophysical processes include radiative transitions in which the excited molecule emits light in the form of fluorescence or phosphorescence and returns to the ground state, and intramolecular non-radiative transitions in which some or all of the energy of the absorbed photon is ultimately converted to heat.

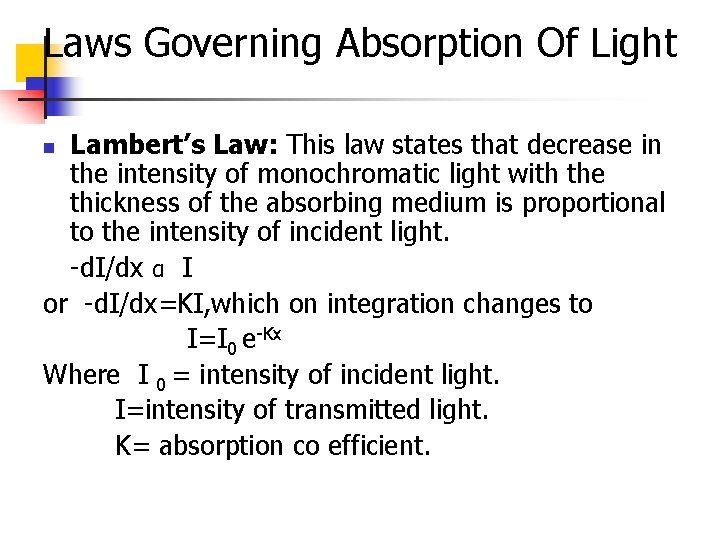
Laws Governing Absorption Of Light Lambert’s Law: This law states that decrease in the intensity of monochromatic light with the thickness of the absorbing medium is proportional to the intensity of incident light. -d. I/dx α I or -d. I/dx=KI, which on integration changes to I=I 0 e-Kx Where I 0 = intensity of incident light. I=intensity of transmitted light. K= absorption co efficient. n

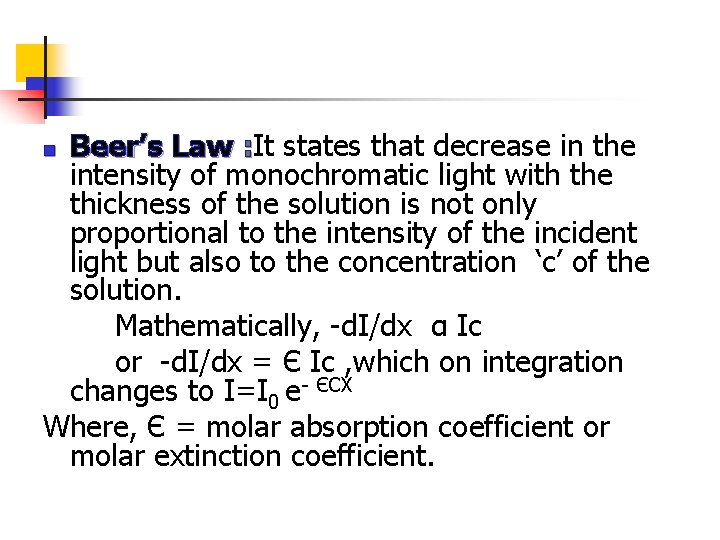
Beer’s Law : It states that decrease in the intensity of monochromatic light with the thickness of the solution is not only proportional to the intensity of the incident light but also to the concentration ‘c’ of the solution. Mathematically, -d. I/dx α Ic or -d. I/dx = Є Ic , which on integration changes to I=I 0 e- ЄCX Where, Є = molar absorption coefficient or molar extinction coefficient. n

Laws governing Photochemistry n Grotthus-Draper Law: Only the light which is absorbed by a molecule can be effective in producing photochemical changes in the molecule. n Stark-Einstein’s Law ( Second Law of Photochemistry): It states that for each photon of light absorbed by a chemical system, only one molecule is activated for a photochemical reaction. The energy absorbed by one mole of the reacting molecules is given by E=Nhv. This energy is called one einstein.

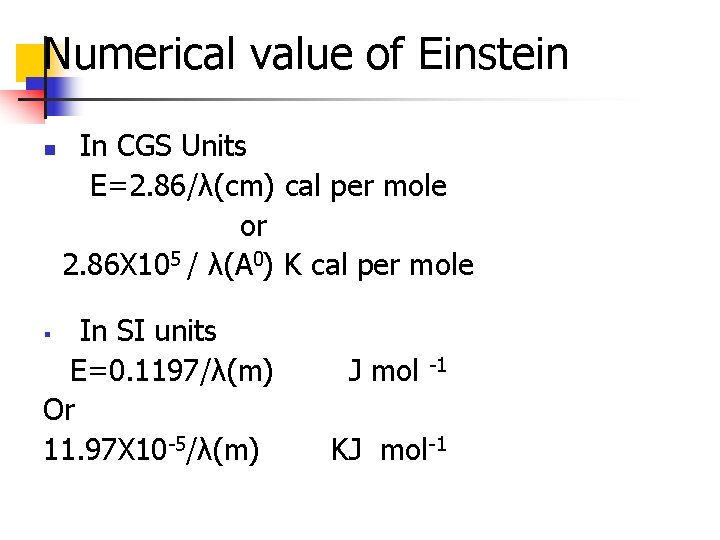
Numerical value of Einstein n In CGS Units E=2. 86/λ(cm) cal per mole or 2. 86 X 105 / λ(A 0) K cal per mole In SI units E=0. 1197/λ(m) Or 11. 97 X 10 -5/λ(m) § J mol -1 KJ mol-1

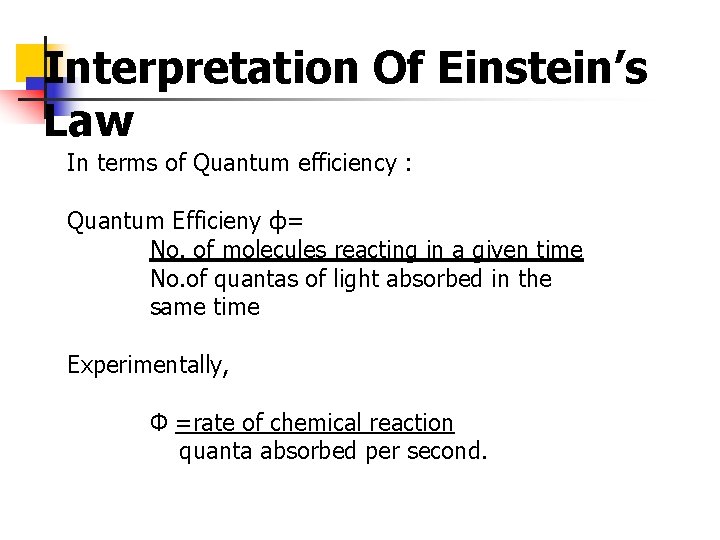
Interpretation Of Einstein’s Law In terms of Quantum efficiency : Quantum Efficieny ф= No. of molecules reacting in a given time No. of quantas of light absorbed in the same time Experimentally, Ф =rate of chemical reaction quanta absorbed per second.

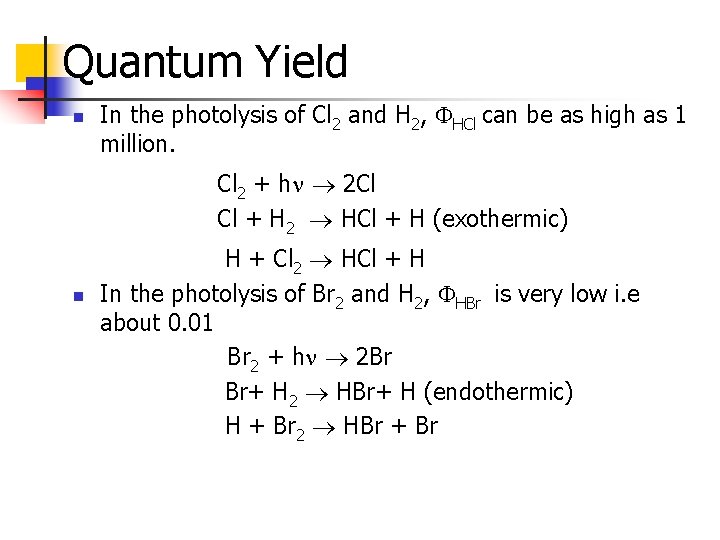
Quantum Yield n In the photolysis of Cl 2 and H 2, HCl can be as high as 1 million. Cl 2 + h 2 Cl Cl + H 2 HCl + H (exothermic) n H + Cl 2 HCl + H In the photolysis of Br 2 and H 2, HBr is very low i. e about 0. 01 Br 2 + h 2 Br Br+ H 2 HBr+ H (endothermic) H + Br 2 HBr + Br

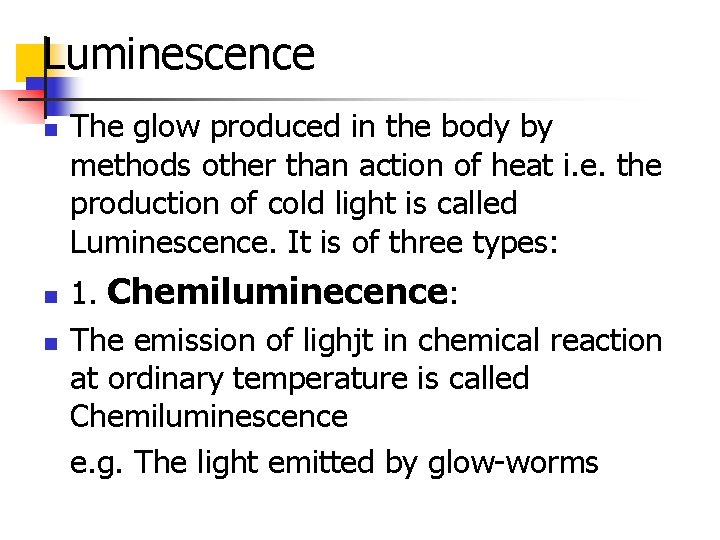
Luminescence n n n The glow produced in the body by methods other than action of heat i. e. the production of cold light is called Luminescence. It is of three types: 1. Chemiluminecence: The emission of lighjt in chemical reaction at ordinary temperature is called Chemiluminescence e. g. The light emitted by glow-worms

Fluorescence: Certain substances when exposed to light or certain other radiations absorb the energy and then immediately start re-emitting the energy. Such substances are called fluorescent substances and the phenomenon is called fluorescence. e. g Organic dyes such as eosin, fluorescein etc. vapour of sodium, mercury, iodine etc. Phosphorescence: There are certain substances which continue to glow for some time even after the external light is cut off. Thus, phosphorescence is a slow fluorescence.

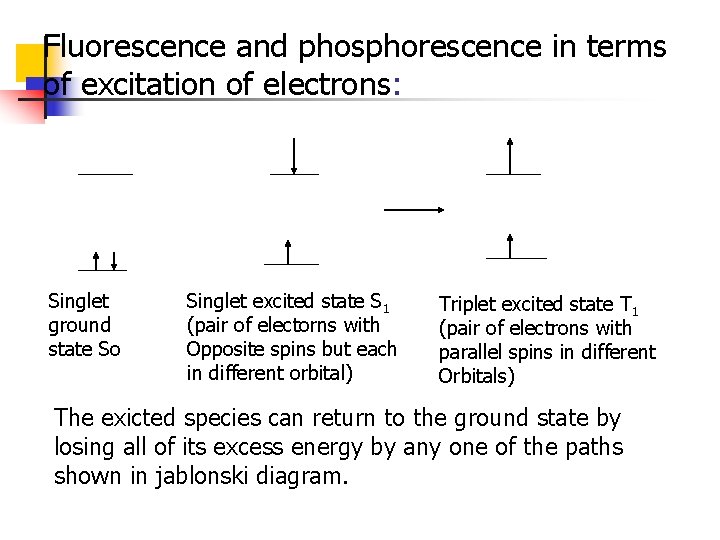
Fluorescence and phosphorescence in terms of excitation of electrons: Singlet ground state So Singlet excited state S 1 (pair of electorns with Opposite spins but each in different orbital) Triplet excited state T 1 (pair of electrons with parallel spins in different Orbitals) The exicted species can return to the ground state by losing all of its excess energy by any one of the paths shown in jablonski diagram.

Photosensitisation n § Photosensitized reactions: An electronically excited molecule can transfer its energy to a second species which then undergoes a photochemical process even though it was not itself directly excited. Mercury acting as a photosensitizer: Hg+hv Hg*+H 2 Hg+2 H Chlorophyll acting as a photosensitizer CO 2+H 2 O+hv chlorophyll 1/6(C 6 H 12 O 6)+O 2

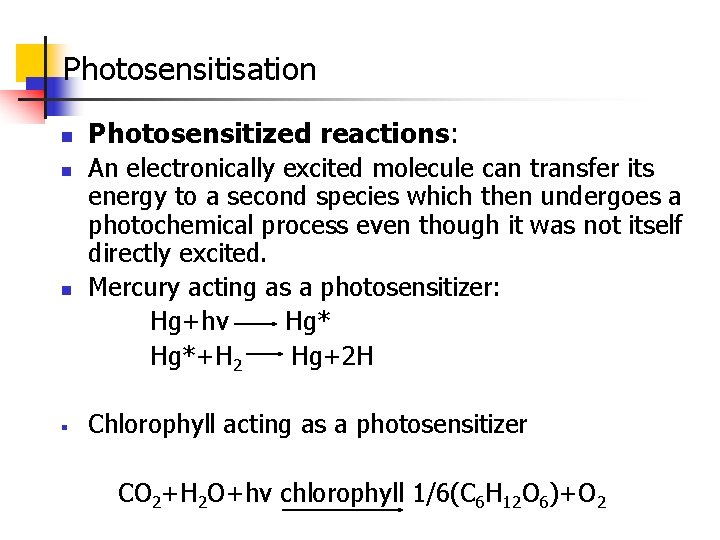
Photochemical Equilibrium A light Thermal B Rate of forward reaction α Iabs=k 1 Iabs Rate of the backward reaction α[B]=k 2[B] At Equiliubrium, Rate of forward reaction=Rate of backward reaction
![K 1 Iabsk 2B or K 2k 1IabsB or KI absB Where KK 2k K 1 Iabs=k 2[B] or K 2/k 1=Iabs/[B] or K=I abs/[B] Where K=K 2/k](https://slidetodoc.com/presentation_image_h2/57de4df3390c3e2f7cb60fefe89751e3/image-15.jpg)
K 1 Iabs=k 2[B] or K 2/k 1=Iabs/[B] or K=I abs/[B] Where K=K 2/k 1 is the photochemical equlibrium constant

Ozone : O 3 A gas composed of three atoms of oxygen bluish gas that is harmful to breathe Nearly 90% of the Earth's ozone is in the stratosphere and is referred to as the ozone layer Ozone absorbs a band of ultraviolet radiation called UVB



Ozone-Depleting Substance(s) (ODS): CFCs, HCFCs, halons, methyl bromide, carbon tetrachloride, and methyl chloroform.

Various sources

Effects of OLD Skin Cancer (melanoma and nonmelanoma) -- Premature aging of the skin and other skin problems -- Cataracts and other eye damage -- Immune system suppression

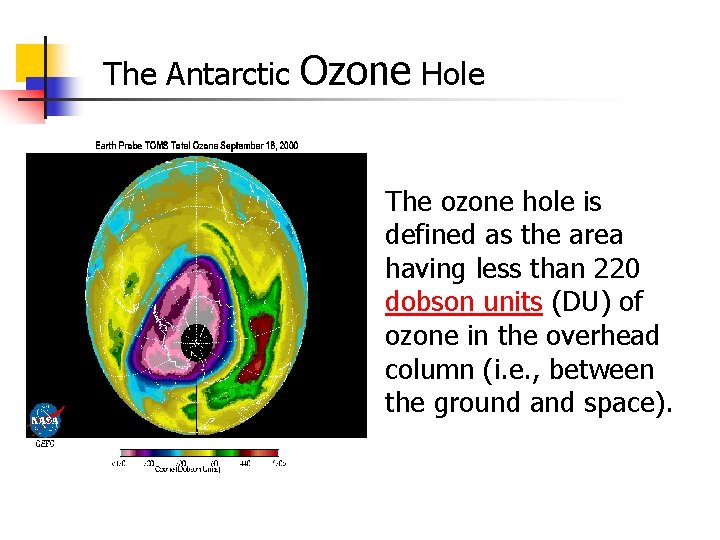
The Antarctic Ozone Hole The ozone hole is defined as the area having less than 220 dobson units (DU) of ozone in the overhead column (i. e. , between the ground and space).

Control Measures 1. Make sure that technicians working on your car air conditioner, home air conditioner, or refrigerator are certified by an EPA approved program to recover the refrigerant (this is required by law). 2. Have your car and home air conditioner units and refrigerator checked for leaks. When possible, repair leaky air conditioning units before refilling them.

3. Keep your automobile well tuned and maintained. 4. Carpool, use mass transit, walk, bicycle, and/or reduce driving, especially on hot summer days. 5. Be careful not to spill gasoline when filling up your car or gasoline-powered lawn and garden equipment. During the summer, fill your gas tank during the cooler evening hours.

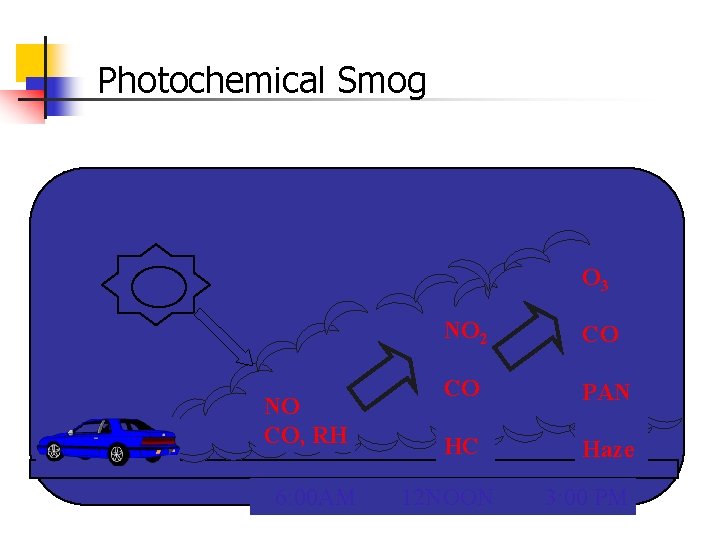
Ground level Ozone Formation Secondary Pollutant VOCs+ NOx Ozone In presence of sunlight Ozone + NOX + HCs Smog (haze)

Photochemical Smog O 3 NO CO, RH 6: 00 AM NO 2 CO CO PAN HC Haze 12 NOON 3: 00 PM

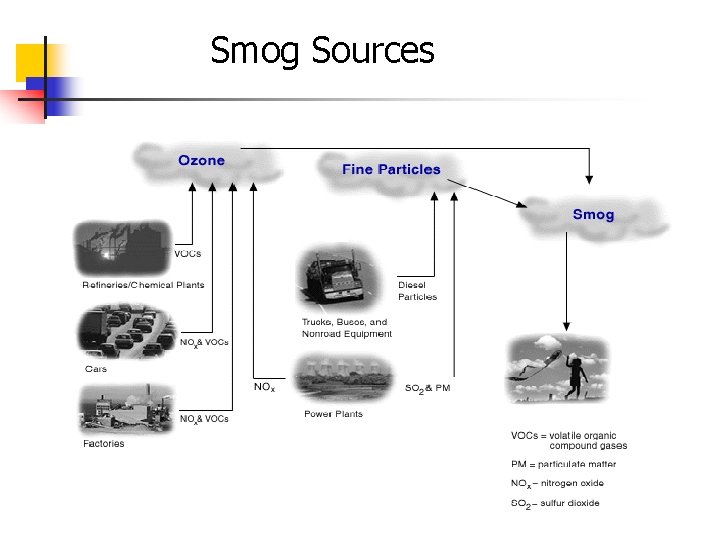
Smog Sources

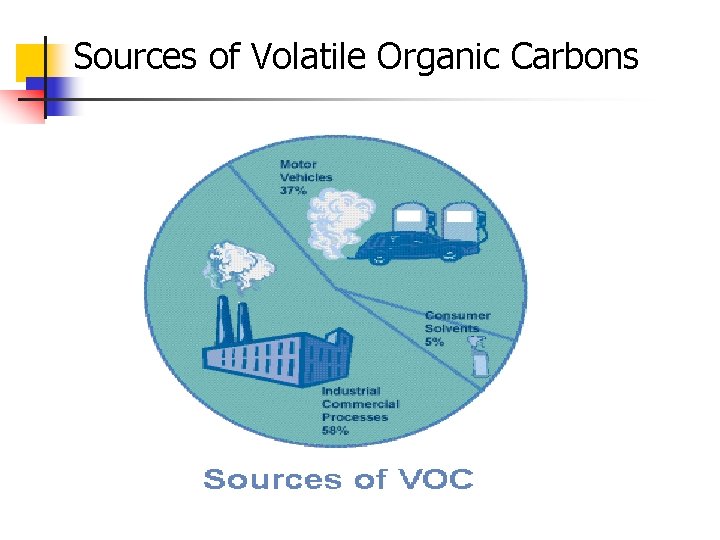
Sources of Volatile Organic Carbons

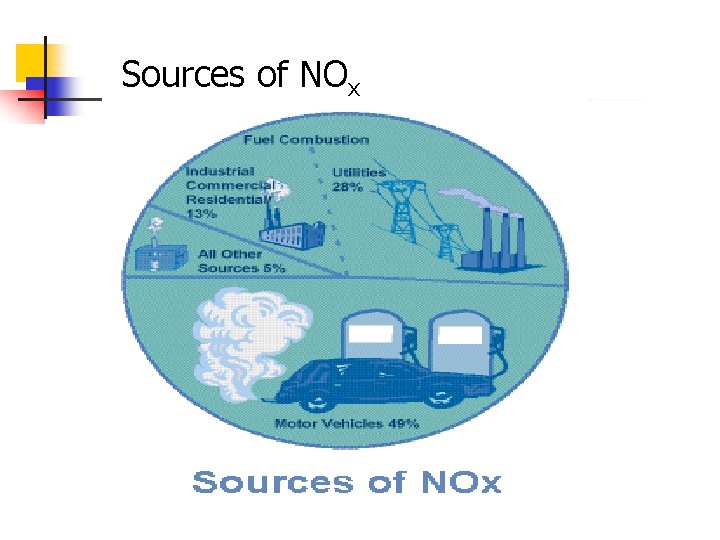
Sources of NOx
 Asc college panvel
Asc college panvel Savitribai phule organization
Savitribai phule organization Usha mehta savitribai phule
Usha mehta savitribai phule Law of photochemistry
Law of photochemistry Photochemistry
Photochemistry Photochemistry
Photochemistry Laws of photochemistry
Laws of photochemistry Photochemistry of vision
Photochemistry of vision Mahatma gandhi transformational leader
Mahatma gandhi transformational leader Fact file of mahatma gandhi
Fact file of mahatma gandhi Bigfun be
Bigfun be 2 oct 1869
2 oct 1869 Cuando nacio mahatma gandhi
Cuando nacio mahatma gandhi Mahatma gandhi bibliografia
Mahatma gandhi bibliografia Eulogy of mahatma gandhi
Eulogy of mahatma gandhi Cuantos hijos tuvo mahatma gandhi
Cuantos hijos tuvo mahatma gandhi Mahatma gandhi short note
Mahatma gandhi short note Explain gandhian philosophy of wealth management
Explain gandhian philosophy of wealth management I due fanciulli pascoli
I due fanciulli pascoli Mahatma gandhi university library
Mahatma gandhi university library Asd college college readiness program
Asd college college readiness program Early college high school at midland college
Early college high school at midland college Scottish police college
Scottish police college College oeben
College oeben Niagara college libraries
Niagara college libraries Castleknock community college evening classes
Castleknock community college evening classes Websmart.smccd
Websmart.smccd Eton college motto
Eton college motto Cbs college publishing
Cbs college publishing Tallinn health care college
Tallinn health care college