Low dose Aspirin for Primary Prevention of Cardiovascular















- Slides: 27

Low- dose Aspirin for Primary Prevention of Cardiovascular Events in Japanese Patients 60 Years or Older with Atherosclerotic Risk Factors Nicholas Leo JAMA. 2014; 312(23): 2510 -2520. doi: 10. 1001/jama. 2014. 15690

Case Summary • 64 Japanese female with DM, HTN, HLD presents for routine follow up. • PMHx: DM, HTN, HLD • Meds: metformin 500 mg BID, lisinopril 40 mg daily, lipitor 20 mg daily • Non-smoker • BP 130/80 • Hb. A 1 c 7. 0%, LDL 80 mg/dl • She recently read about the benefits of aspirin on a health magazine and asked if she can be put on aspirin.

Background • In 2009, the Antithrombotic Trialists’ Collaboration (ATTC) reviewed the benefit-risk profile of low-dose aspirin for the primary prevention of vascular disease in a meta-analysis of 6 primary prevention trials. • Use of low-dose aspirin was associated with a 12% proportional reduction in serious vascular events compared with no aspirin (annual event rate, 0. 51% for aspirin and 0. 57% for no aspirin; P =. 001), mainly due to a reduction in nonfatal myocardial infarction.

Background • Aspirin demonstrated little effect on death from CHD (RR 0. 95, 95% CI 0. 78 -1. 15) • No significant effect on stroke (RR 0. 95, 95% CI 0. 85 -1. 06 • Aspirin increased major gastrointestinal and extracranial bleeding compared with control (annual increase, 0. 10% for aspirin and 0. 07% for control; P <. 001).

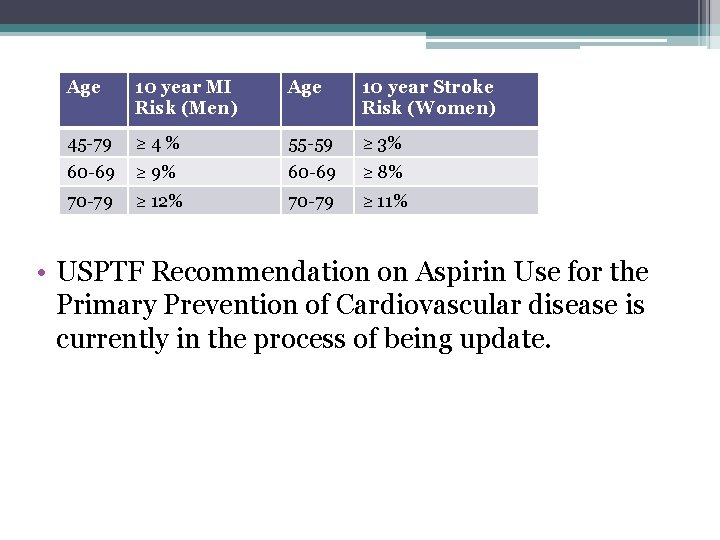
USPTF Recommendations 2009 • Aspirin is recommended for men age 45– 79 to reduce risk of myocardial infarction (MI) when a net benefit is present • Aspirin is recommended for women age 55– 79 to reduce risk of ischemic stroke when a net benefit is present. • The USPSTF recommends AGAINST the use of aspirin for the primary prevention of MI in men less than age 45 or stroke in women less than age 55. • The USPSTF found the evidence insufficient to recommend for or against the use of aspirin for MI or stroke reduction in men and women age 80 and older.

Age 10 year MI Risk (Men) Age 10 year Stroke Risk (Women) 45 -79 ≥ 4% 55 -59 ≥ 3% 60 -69 ≥ 9% 60 -69 ≥ 8% 70 -79 ≥ 12% 70 -79 ≥ 11% • USPTF Recommendation on Aspirin Use for the Primary Prevention of Cardiovascular disease is currently in the process of being update.

Background • There is a paucity of data in Asian populations, in which hemoorhagic stroke risk tendes to be higher than in Western population, and thus this population is of particular interest.

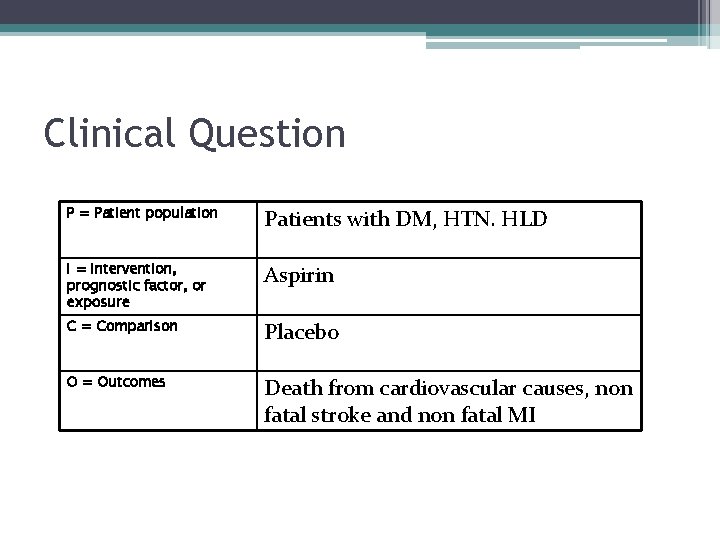
Clinical Question P = Patient population Patients with DM, HTN. HLD I = Intervention, prognostic factor, or exposure Aspirin C = Comparison Placebo O = Outcomes Death from cardiovascular causes, non fatal stroke and non fatal MI

Clinical Question • In patient with DM, HTN, HLD, does the use of aspirin as primary prophylaxis reduce risk of death from cardiovascular causes, non-fatal stroke and non-fatal MI?

Methods • Design: Multicenter, open-label, randomized parallel-group trial • Setting: 1007 clinics in Japan • Follow up period: 6. 5 years

Patient Population • Inclusion criteria: Age 60 to 85 years and had not been diagnosed with atherosclerotic disease • Exclusion criteria: - History of CAD or CVA (including TIA), atherosclerotic disease requiring surgery or intervention, or atrial fibrillation (confirmed or suspected). - Patients with peptic ulcer or conditions associated with bleeding (eg, von Willebrand disease) and those with serious blood abnormalities (eg, clotting factor deficiencies) were also excluded. - Patients with aspirin-sensitive asthma or those with a history of hypersensitivity to aspirin or salicylic acid could not participate - Patients who were receiving antiplatelet agents, anticoagulants, or long-term treatment with nonsteroidal anti-inflammatory drugs.

Methods • Randomization was stratified by the 3 underlying disease risk factors for atherosclerotic events (hypertension, dyslipidemia, or diabetes). • Seven strata were used to account for all the different combinations • At baseline and at each annual study assessment, disease outcomes, adverse events, adherence with treatment (self-reported by patients), blood pressure, serum lipids, blood glucose, smoking status, and body weight were evaluated

Methods • Follow-up of patients ceased in the event of death or withdrawal of consent. • If cause of death was unclear, it was established by obtaining the death certificate with permission from the Japanese government. • Study end points were assessed centrally and biannually by an expert, multidisciplinary event adjudication committee that was blinded to treatment assignments

Primary Endpoint • Death from cardiovascular causes (myocardial infarction, stroke, and other cardiovascular causes), nonfatal stroke (ischemic or hemorrhagic, including undefined cerebrovascular events), and nonfatal myocardial infarction

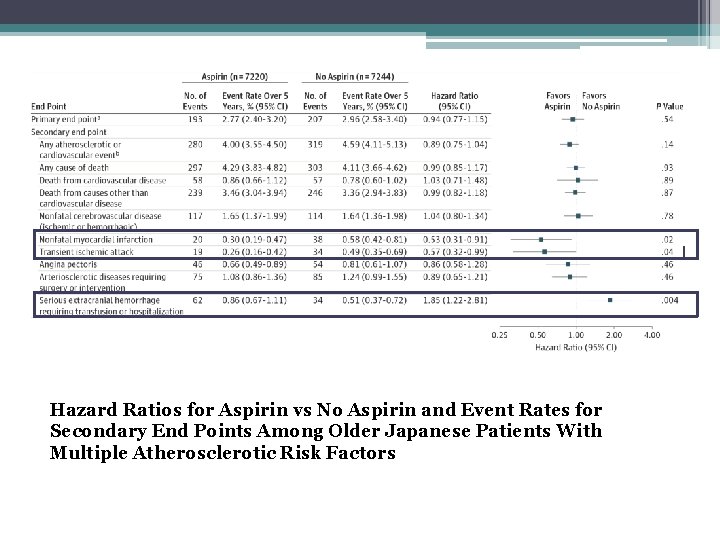
Secondary Endpoint • Individual end points • Serious extracranial hemorrhage requiring transfusion or hospitalization

Statistical Analysis • Initially, a sample size of 10, 000 patients was determined to be sufficient. • However, a reduced incidence of primary outcome events was observed at the first annual review. Sample size was then increased to 14, 960 • Hazard ratios (HRs) were calculated using the Cox proportional hazards model and 95% CIs were determined. • All analysis were assessed using a modified intention-to-treat population


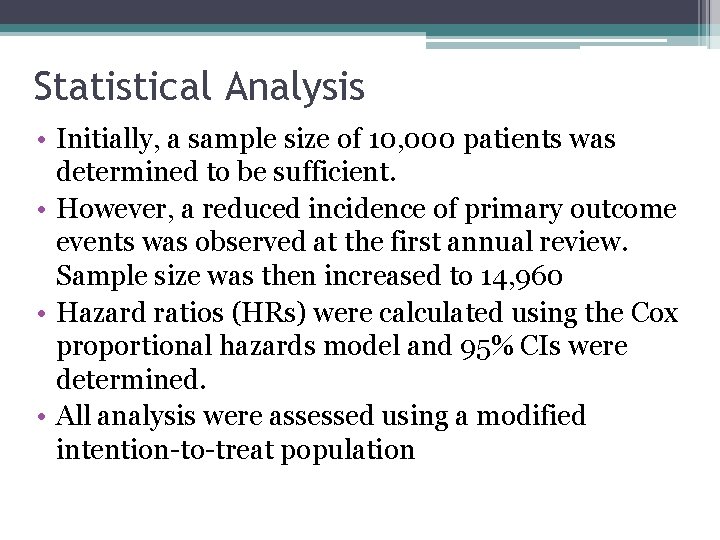
Baseline Characteristics for Japanese Patients Receiving Aspirin or No Aspirin (Modified Intention-to-Treat Population)

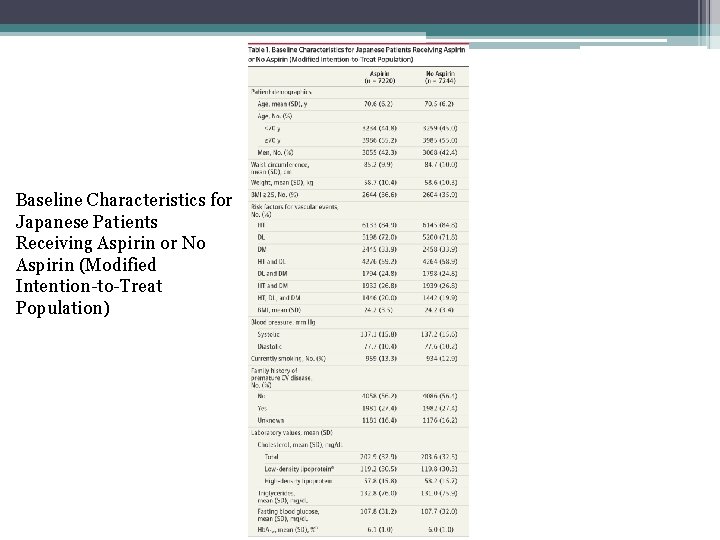
Time to Primary End Point Composite Event Among Older Japanese Patients With Multiple Atherosclerotic Risk Factors Receiving Aspirin vs No Aspirin.

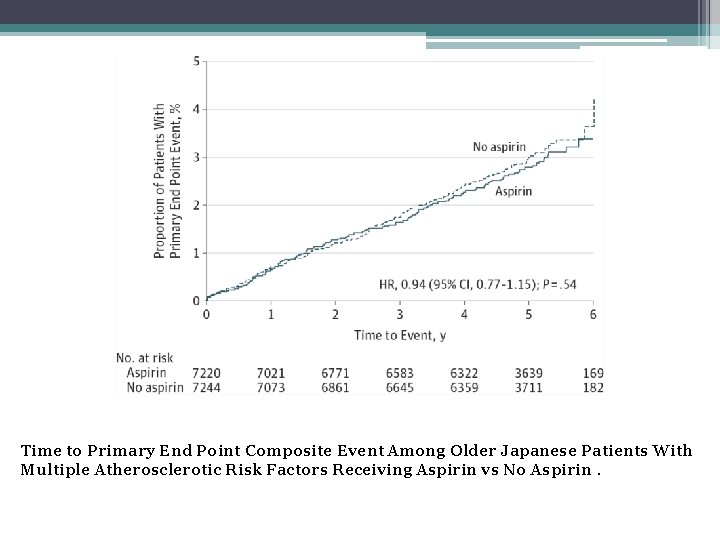
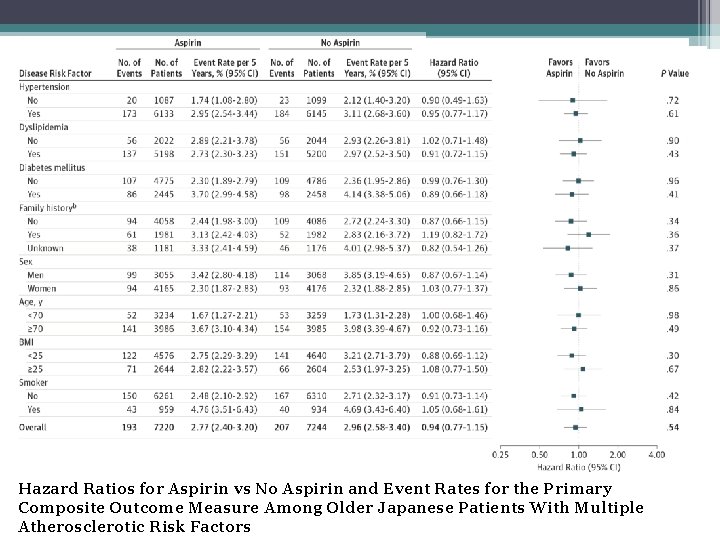
Hazard Ratios for Aspirin vs No Aspirin and Event Rates for the Primary Composite Outcome Measure Among Older Japanese Patients With Multiple Atherosclerotic Risk Factors

Hazard Ratios for Aspirin vs No Aspirin and Event Rates for Secondary End Points Among Older Japanese Patients With Multiple Atherosclerotic Risk Factors


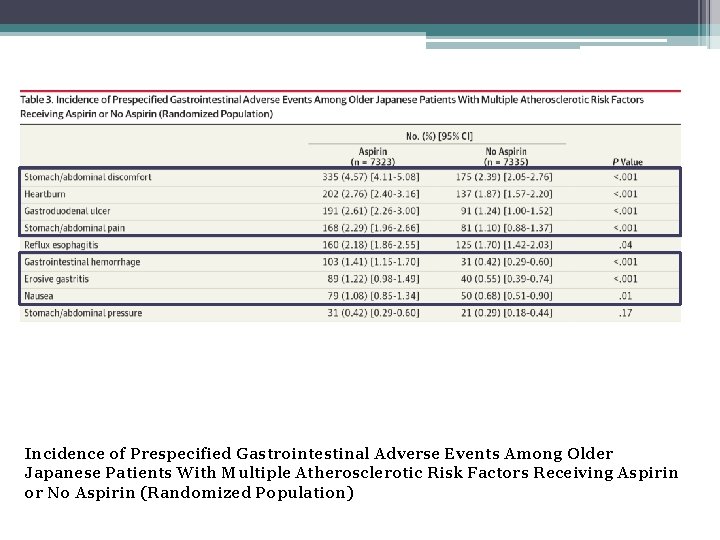
Incidence of Prespecified Gastrointestinal Adverse Events Among Older Japanese Patients With Multiple Atherosclerotic Risk Factors Receiving Aspirin or No Aspirin (Randomized Population)

Are the results valid? Strengths • Allocation concealment • Randomized (Baseline Characteristics similar in both groups)

Are the results valid? Weaknesses • Patients and physicians in this trial were not blinded to treatment assignment. • No placebo • Inadequate power. Study was designed to detect 20% relative difference which is larger than 12% reduction seen in pooled primary prevention studies. • Modified Intention to treat analysis • Decreased adherence in aspirin group (76% in year 5) and increasing uptake in the no aspirin group (reaching 10 % in year 5)

Does the results apply to my patient? • Yes, she meets inclusion criteria. • Based on USPTF guidelines, aspirin will not be recommended as her 10 year stroke risk is 7. 5 % (<8%) • Based on this trial, I will not recommend aspirin as the benefit of aspirin, if any, is not very strong.

Conclusion • Benefit of aspirin in primary prevention of cardiovascular events is not as strong as secondary prevention. • Other primary prevention studies using aspirin (ARRIVE, ASCEND, ASPREE ) are in progress.
 Primary prevention secondary prevention tertiary prevention
Primary prevention secondary prevention tertiary prevention Dobutamine low dose
Dobutamine low dose Hologic's low dose 3d mammography
Hologic's low dose 3d mammography Low dose naltrexone walgreens
Low dose naltrexone walgreens Middle = low + (high - low) / 2
Middle = low + (high - low) / 2 Communication style bias
Communication style bias Low accuracy low precision
Low accuracy low precision Low voltage hazards
Low voltage hazards Tertiary prevention example
Tertiary prevention example Primary prevention examples
Primary prevention examples Conclusion of malaria
Conclusion of malaria Functional groups in aspirin
Functional groups in aspirin Cooh functional group
Cooh functional group Mechanism of action of asprin
Mechanism of action of asprin Trombolitis
Trombolitis Molecular mass
Molecular mass Solubilization of aspirin
Solubilization of aspirin Lydia glaw
Lydia glaw Oil of wintergreen synthesis
Oil of wintergreen synthesis Making aspirin
Making aspirin Aspirin back titration
Aspirin back titration Principle of assay of aspirin
Principle of assay of aspirin Mechanism of action of aspirin
Mechanism of action of aspirin Aspirin mechanism of action
Aspirin mechanism of action Identification of aspirin
Identification of aspirin Polarity of aspirin acetaminophen ibuprofen caffeine
Polarity of aspirin acetaminophen ibuprofen caffeine Aspirin brausepulver
Aspirin brausepulver Jenis pulvis
Jenis pulvis