LIMPORTANZA DELLA SCELTA DEL METODO ANALITICO NELLA CLEANING








- Slides: 16

L’IMPORTANZA DELLA SCELTA DEL METODO ANALITICO NELLA CLEANING VALIDATION Damiana Gentili Qualified Person/QU Director 09/05/2019

AGENDA • Regulatory framework • Specific vs non-specific • Visual inspection • Selection criteria for analytical methods & case history • Analytical Validation overview • Opportunities

FDA OUTCOMES ON ANALYTICAL METHODS FOR CLEANING VALIDATION • Failure to adequately validate written procedures for the cleaning and maintenance of equipment

ITALY

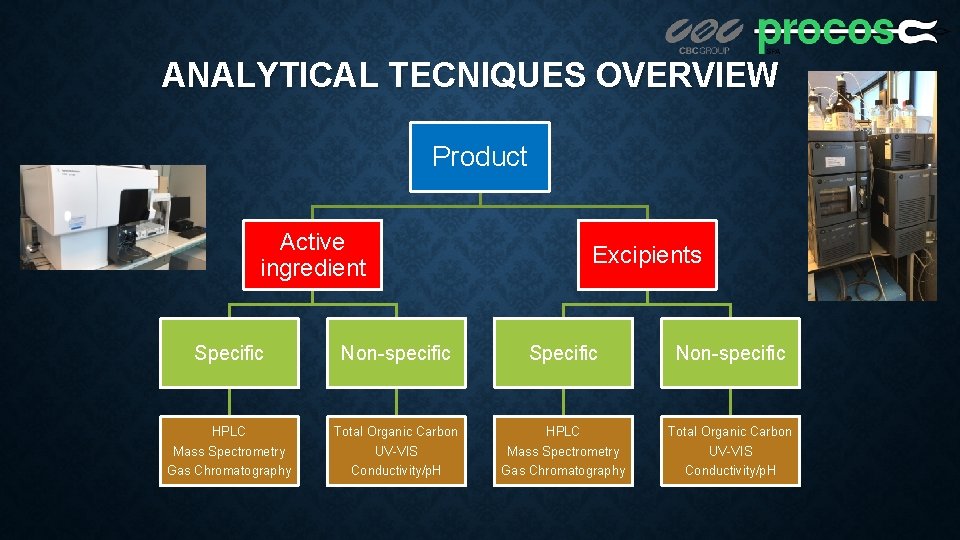
ANALYTICAL TECNIQUES OVERVIEW Product Active ingredient Excipients Specific Non-specific HPLC Mass Spectrometry Gas Chromatography Total Organic Carbon UV-VIS Conductivity/p. H

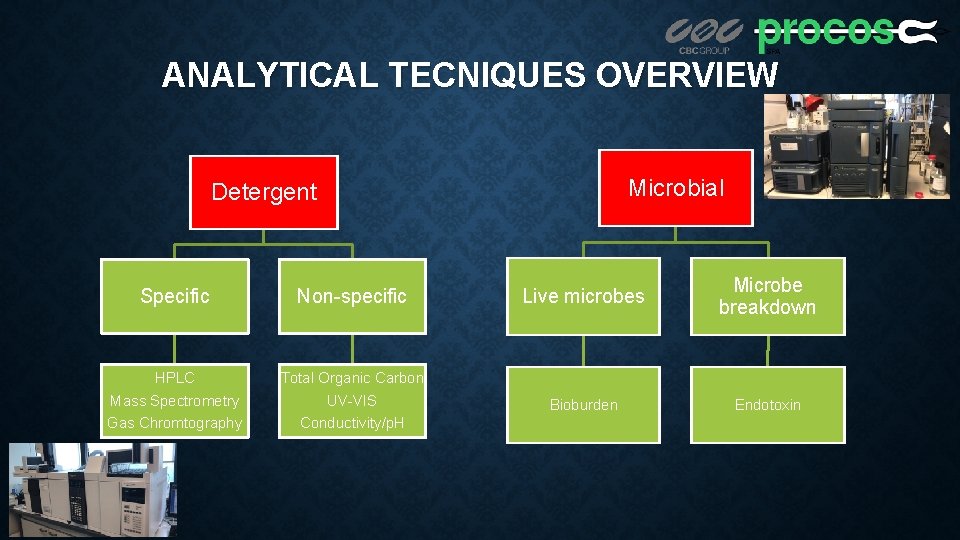
ANALYTICAL TECNIQUES OVERVIEW Microbial Detergent Specific Non-specific Live microbes Microbe breakdown HPLC Mass Spectrometry Gas Chromtography Total Organic Carbon UV-VIS Conductivity/p. H Bioburden Endotoxin

DETECTION OF RESIDUES: FROM 1992 UP TO DATE… SPECIFIC VS. NON-SPECIFIC

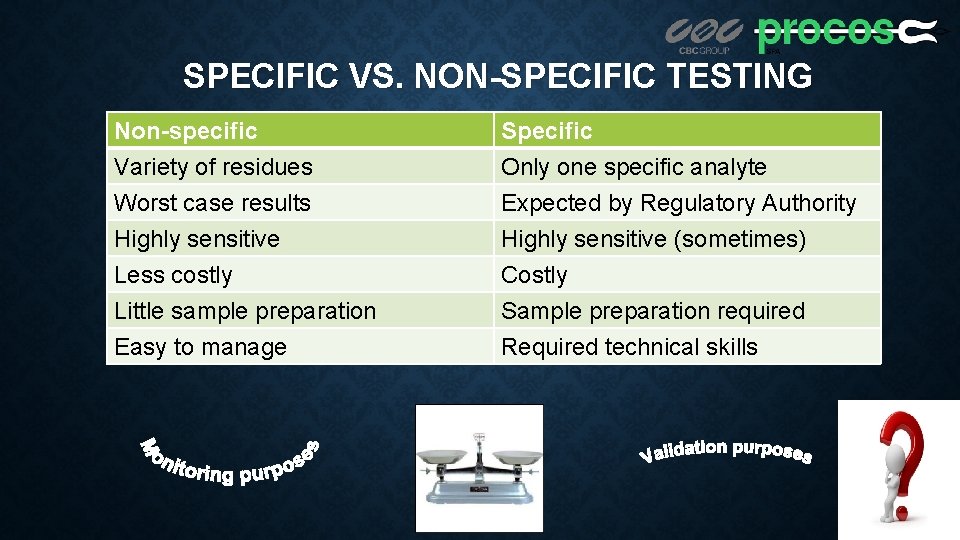
SPECIFIC VS. NON-SPECIFIC TESTING Non-specific Variety of residues Worst case results Highly sensitive Specific Only one specific analyte Expected by Regulatory Authority Highly sensitive (sometimes) Less costly Little sample preparation Easy to manage Costly Sample preparation required Required technical skills

VISIBLE RESIDUE § This testing is designed to make observations directly from the clean equipment for quick turnaround § Visual inspection using a visible residue limit (VRL) can monitor equipment cleaning efficiency § The VRL is the level below which the residue of interest is not visible to the equipment Characteristics • A VRL inspection is non-specific • Any visible residue is considered unacceptable § Several of its applications and associated risks have been demonstrate § Since this is a limit test, the detection limit is the primary validation parameter. § Precision should be also determined by using multiple observers in order to minimise subjectivity of the observers • Subjectivity and training of the inspectors is the primary concern with this process Results from validation activity can be used to establish the difference between the Analytical Residue Limit and the Validation Residue Limit?

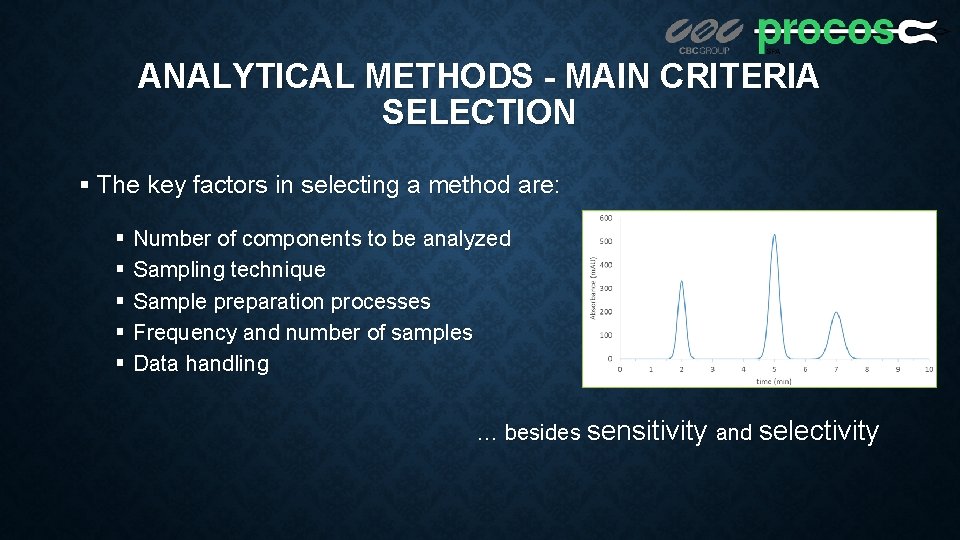
ANALYTICAL METHODS - MAIN CRITERIA SELECTION § The key factors in selecting a method are: § § § Number of components to be analyzed Sampling technique Sample preparation processes Frequency and number of samples Data handling … besides sensitivity and selectivity

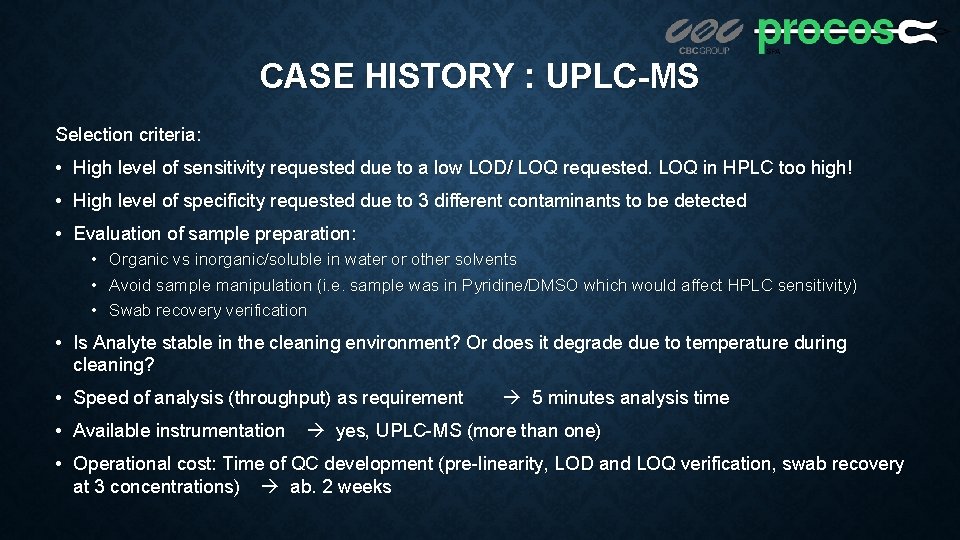
CASE HISTORY : UPLC-MS Selection criteria: • High level of sensitivity requested due to a low LOD/ LOQ requested. LOQ in HPLC too high! • High level of specificity requested due to 3 different contaminants to be detected • Evaluation of sample preparation: • Organic vs inorganic/soluble in water or other solvents • Avoid sample manipulation (i. e. sample was in Pyridine/DMSO which would affect HPLC sensitivity) • Swab recovery verification • Is Analyte stable in the cleaning environment? Or does it degrade due to temperature during cleaning? • Speed of analysis (throughput) as requirement • Available instrumentation 5 minutes analysis time yes, UPLC-MS (more than one) • Operational cost: Time of QC development (pre-linearity, LOD and LOQ verification, swab recovery at 3 concentrations) ab. 2 weeks

FINAL OUTPUT Chromatographic conditions • The chromatographic procedure may be carried out using: - column: ACQUITY UPLC BEH C 18, 100 × 2. 1 mm, 1. 7 μm or equivalent; - column temperature: 30°C; - Mobile phases: Mobile phase A: 0. 1% formic acid in water (for example transfer 1. 0 ml of formic acid 98% into 1000 ml water); Mobile phase B: 0. 1% formic acid in acetonitrile (for example transfer 1. 0 ml of formic acid 98% into 1000 ml water); - Detection: MS (settings are optimized for Waters Acquity QDa Detector), Single Ion Recording (SIR) Ionization Mode: ESI +; Probe Temp. 600 °C; Capillary 0. 8 k. V; Cone: 15 V; Acquisition Type: Selected Ion Recording (SIR); MASS: 219. 0 amu M 984 N 5 - Injection volume: 1 µl; - Flow rate: 0. 5 ml/min; - Run time: 5 min MASS: 431. 0 amu N 5. 1 MASS: 474. 0 amu

TEST METHOD VALIDATION FOR CV SAMPLES • Testing cleaning validation samples requires a validated method • The extent of validation is dependent upon • the type of method employed • the capabilities of the method • the scientific and regulatory needs of the resulting data • the anticipated outcome of the testing

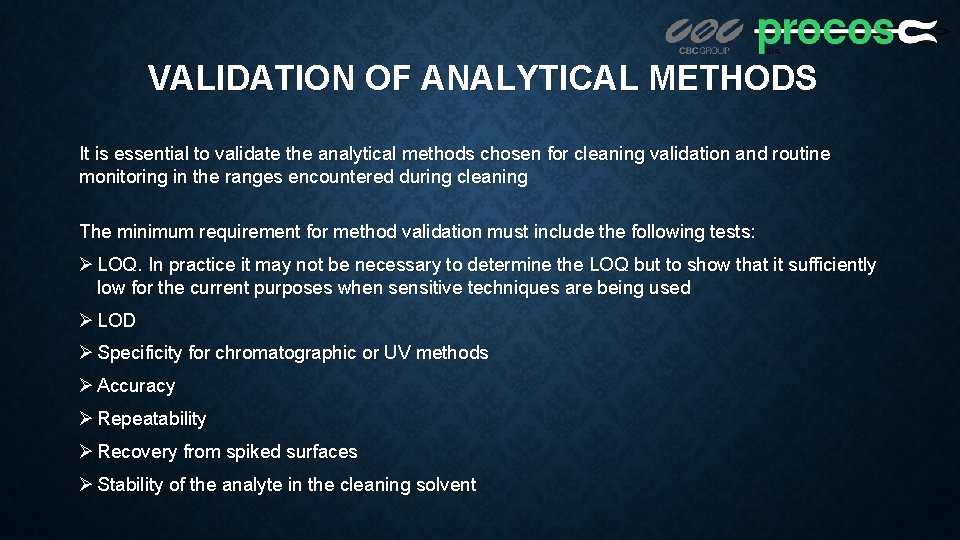
VALIDATION OF ANALYTICAL METHODS It is essential to validate the analytical methods chosen for cleaning validation and routine monitoring in the ranges encountered during cleaning The minimum requirement for method validation must include the following tests: Ø LOQ. In practice it may not be necessary to determine the LOQ but to show that it sufficiently low for the current purposes when sensitive techniques are being used Ø LOD Ø Specificity for chromatographic or UV methods Ø Accuracy Ø Repeatability Ø Recovery from spiked surfaces Ø Stability of the analyte in the cleaning solvent

WHERE WE ARE AS API MANUFACTURER • We use specific analytical method rather than non-specific • We use solvents as cleaning agents and very low use of detergents • We use high level of sophisticated equipment NEW OPPORTUNITIES TO EXPLORE 1. Use non-specific methods/easiest analytical tecniques for monitoring purposes 2. Use detergents to reduce safety issues in handling solvents as cleaning agents 3. Correlate monitoring results with validation data Increase the level of control of cleaning

Tempio del padiglione d'oro Thank you very much gentili@procos. it
 Teoria scelta razionale
Teoria scelta razionale Precisione di un metodo analitico
Precisione di un metodo analitico Eclctico
Eclctico Diferencia entre metodo perpetuo y analitico
Diferencia entre metodo perpetuo y analitico Validazione di un metodo analitico
Validazione di un metodo analitico Ley del coseno vectores
Ley del coseno vectores Metodo analítico
Metodo analítico Analitico sintetico globale
Analitico sintetico globale Vectores coordenadas geograficas
Vectores coordenadas geograficas Conto economico abbreviato
Conto economico abbreviato Codici classroom unife medicina corsi a scelta
Codici classroom unife medicina corsi a scelta Domande a risposta multipla
Domande a risposta multipla Tema della diversità nella letteratura italiana
Tema della diversità nella letteratura italiana Sono esenti dall'applicazione della cmr i traslochi
Sono esenti dall'applicazione della cmr i traslochi Il tema della follia nella letteratura
Il tema della follia nella letteratura Indice tematico tentativo
Indice tematico tentativo Bene non seppi
Bene non seppi































