Light and the Photon The Great Max Planck


- Slides: 10

Light and the Photon The Great Max Planck

A small problem in 1901 • In 1901 most people thought that all of th emajor discoveries in physics had been made. • There were a few loose ends to tie up but that is all- so they thought. • It was well known that light for instance travelled as waves. Light can do the things that all waves do • One of the problems was related to the way in which objects give off light when they are heated

Radiating Heat • Standard bodies are black • Measure the radiation given off by a black body at every frequency of the spectrum at a fixed temperature

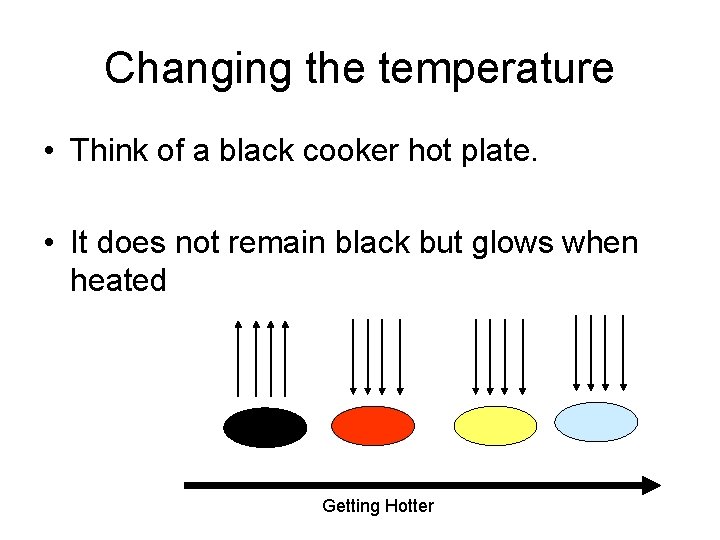
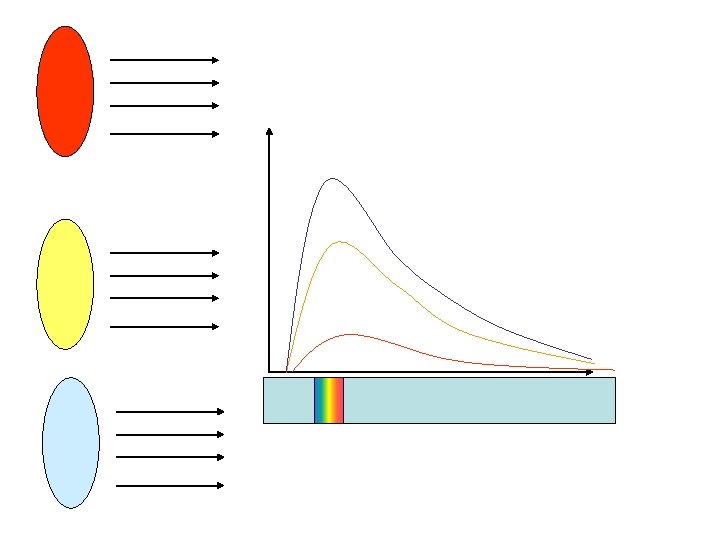
Changing the temperature • Think of a black cooker hot plate. • It does not remain black but glows when heated Getting Hotter


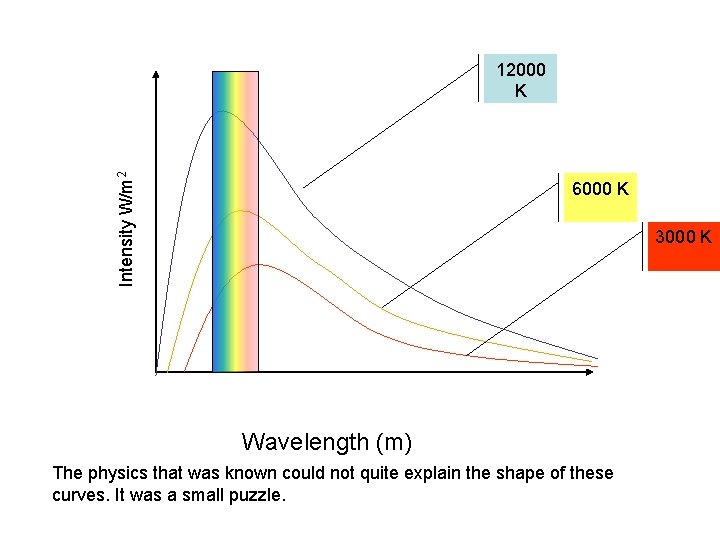
Intensity W/m 2 12000 K 6000 K 3000 K Wavelength (m) The physics that was known could not quite explain the shape of these curves. It was a small puzzle.

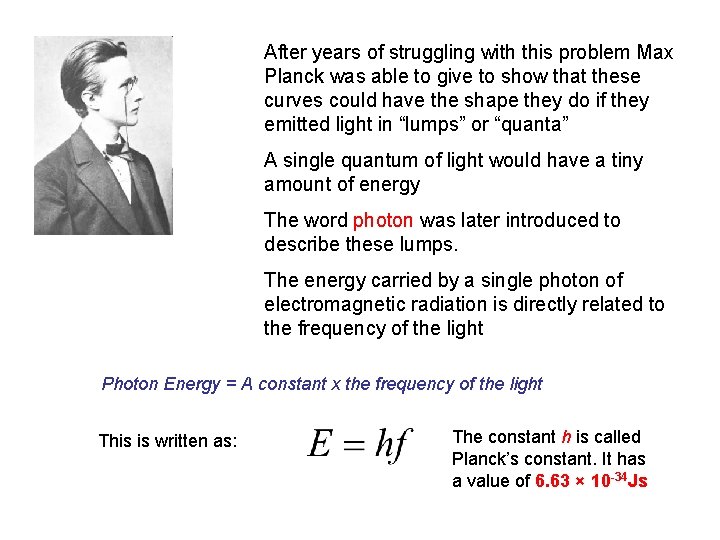
After years of struggling with this problem Max Planck was able to give to show that these curves could have the shape they do if they emitted light in “lumps” or “quanta” A single quantum of light would have a tiny amount of energy The word photon was later introduced to describe these lumps. The energy carried by a single photon of electromagnetic radiation is directly related to the frequency of the light Photon Energy = A constant x the frequency of the light This is written as: The constant h is called Planck’s constant. It has a value of 6. 63 × 10 -34 Js

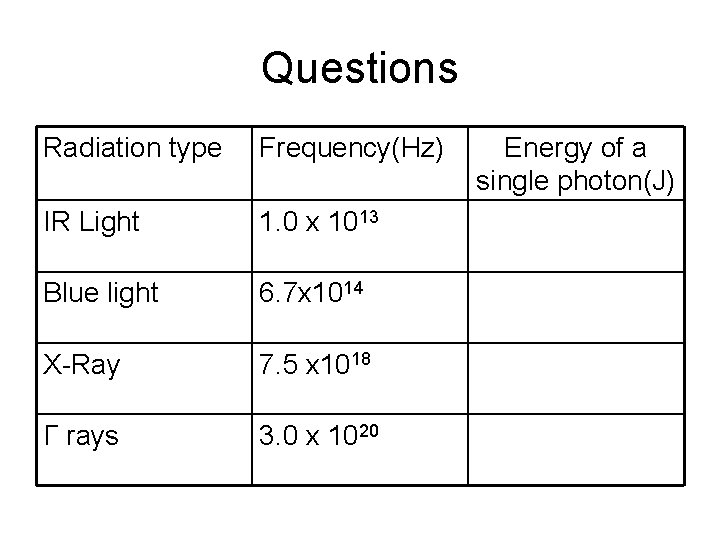
Questions Radiation type Frequency(Hz) IR Light 1. 0 x 1013 Blue light 6. 7 x 1014 X-Ray 7. 5 x 1018 Γ rays 3. 0 x 1020 Energy of a single photon(J)

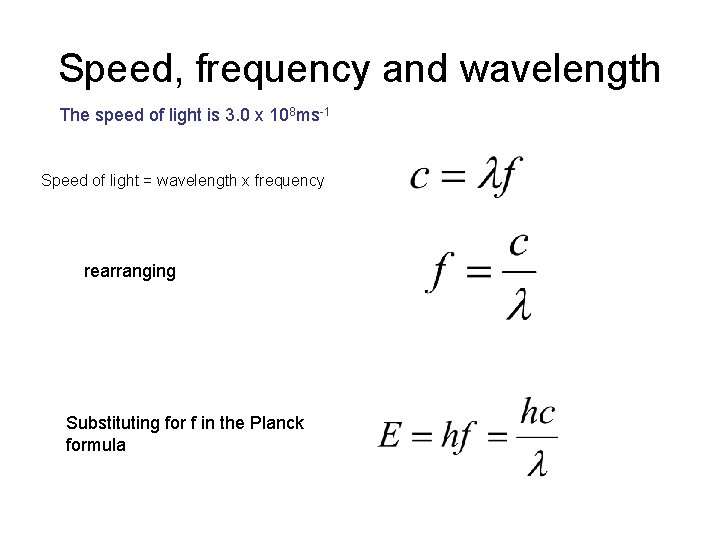
Speed, frequency and wavelength The speed of light is 3. 0 x 108 ms-1 Speed of light = wavelength x frequency rearranging Substituting for f in the Planck formula

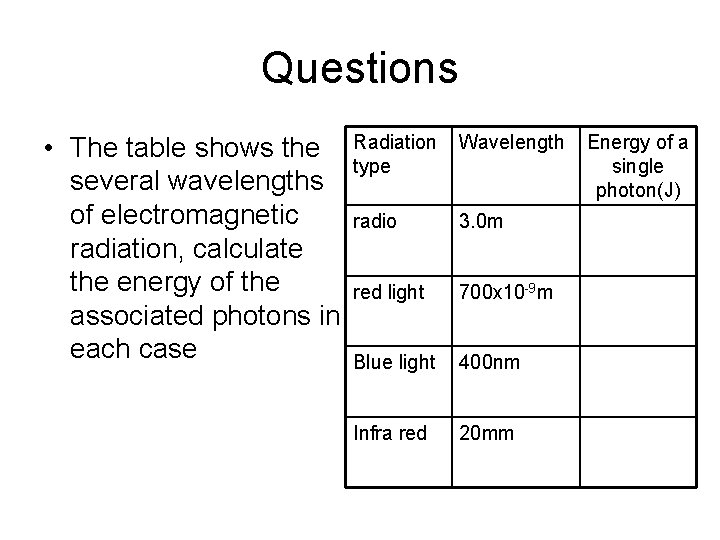
Questions • The table shows the several wavelengths of electromagnetic radiation, calculate the energy of the associated photons in each case Radiation type Wavelength radio 3. 0 m red light 700 x 10 -9 m Blue light 400 nm Infra red 20 mm Energy of a single photon(J)