ISM Astrochemistry Lecture 3 Models History 1950 1972


- Slides: 14

ISM & Astrochemistry Lecture 3

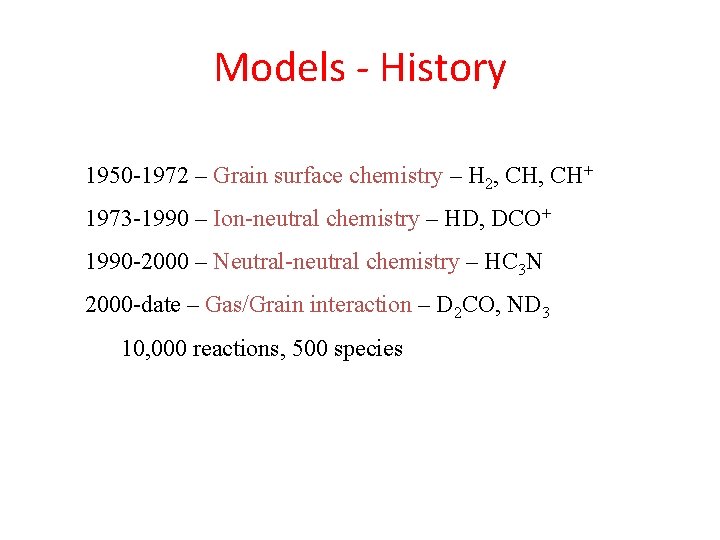
Models - History 1950 -1972 – Grain surface chemistry – H 2, CH+ 1973 -1990 – Ion-neutral chemistry – HD, DCO+ 1990 -2000 – Neutral-neutral chemistry – HC 3 N 2000 -date – Gas/Grain interaction – D 2 CO, ND 3 10, 000 reactions, 500 species

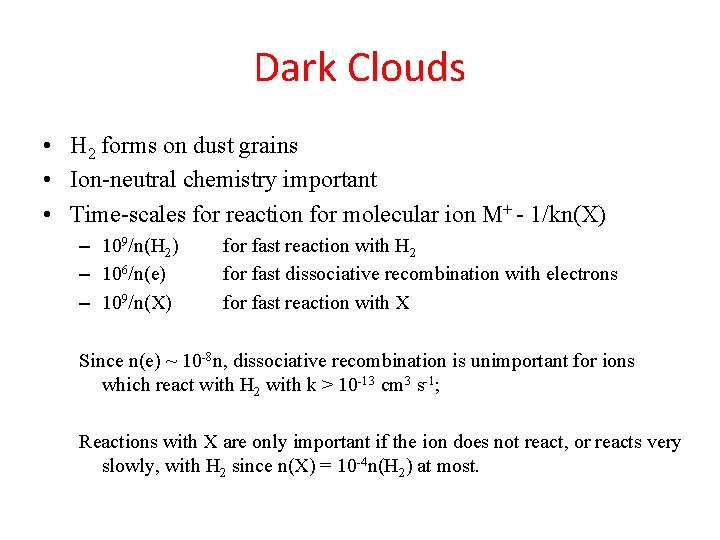
Dark Clouds • H 2 forms on dust grains • Ion-neutral chemistry important • Time-scales for reaction for molecular ion M+ - 1/kn(X) – 109/n(H 2) – 106/n(e) – 109/n(X) for fast reaction with H 2 for fast dissociative recombination with electrons for fast reaction with X Since n(e) ~ 10 -8 n, dissociative recombination is unimportant for ions which react with H 2 with k > 10 -13 cm 3 s-1; Reactions with X are only important if the ion does not react, or reacts very slowly, with H 2 since n(X) = 10 -4 n(H 2) at most.

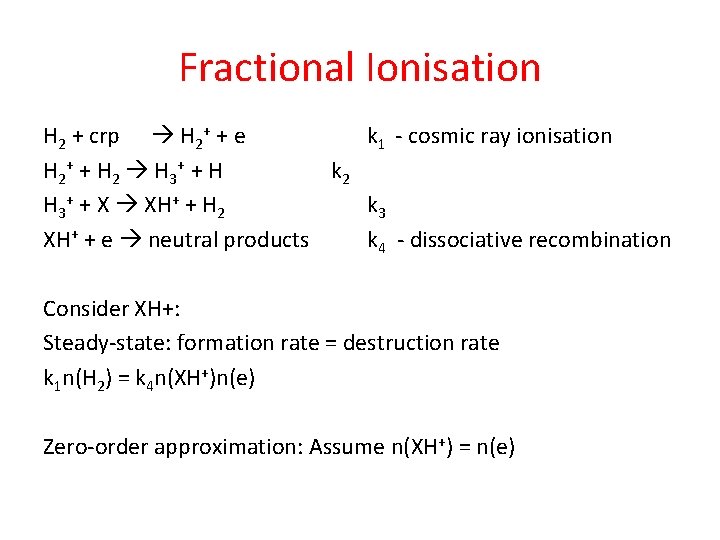
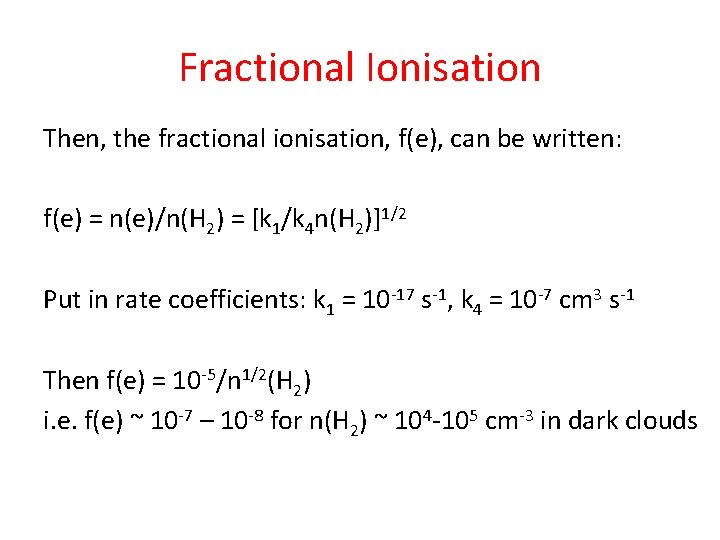
Fractional Ionisation H 2 + crp H 2+ + e k 1 - cosmic ray ionisation H 2 + + H 2 H 3 + + H k 2 H 3+ + X XH+ + H 2 k 3 XH+ + e neutral products k 4 - dissociative recombination Consider XH+: Steady-state: formation rate = destruction rate k 1 n(H 2) = k 4 n(XH+)n(e) Zero-order approximation: Assume n(XH+) = n(e)

Fractional Ionisation Then, the fractional ionisation, f(e), can be written: f(e) = n(e)/n(H 2) = [k 1/k 4 n(H 2)]1/2 Put in rate coefficients: k 1 = 10 -17 s-1, k 4 = 10 -7 cm 3 s-1 Then f(e) = 10 -5/n 1/2(H 2) i. e. f(e) ~ 10 -7 – 10 -8 for n(H 2) ~ 104 -105 cm-3 in dark clouds

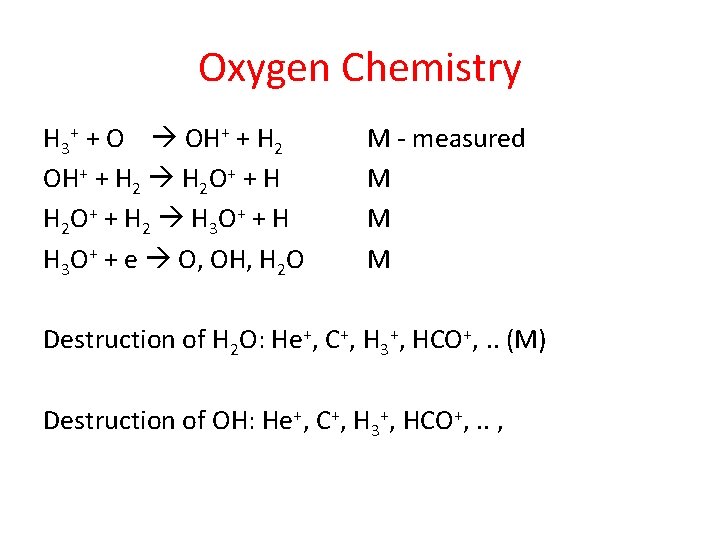
Oxygen Chemistry H 3+ + O OH+ + H 2 O+ + H H 2 O + + H 2 H 3 O + + H H 3 O+ + e O, OH, H 2 O M - measured M M M Destruction of H 2 O: He+, C+, H 3+, HCO+, . . (M) Destruction of OH: He+, C+, H 3+, HCO+, . . ,

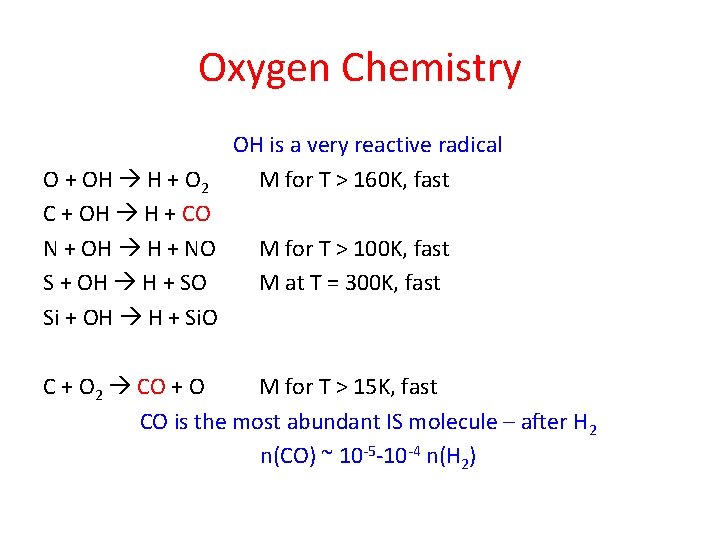
Oxygen Chemistry O + OH H + O 2 C + OH H + CO N + OH H + NO S + OH H + SO Si + OH H + Si. O OH is a very reactive radical M for T > 160 K, fast M for T > 100 K, fast M at T = 300 K, fast C + O 2 CO + O M for T > 15 K, fast CO is the most abundant IS molecule – after H 2 n(CO) ~ 10 -5 -10 -4 n(H 2)

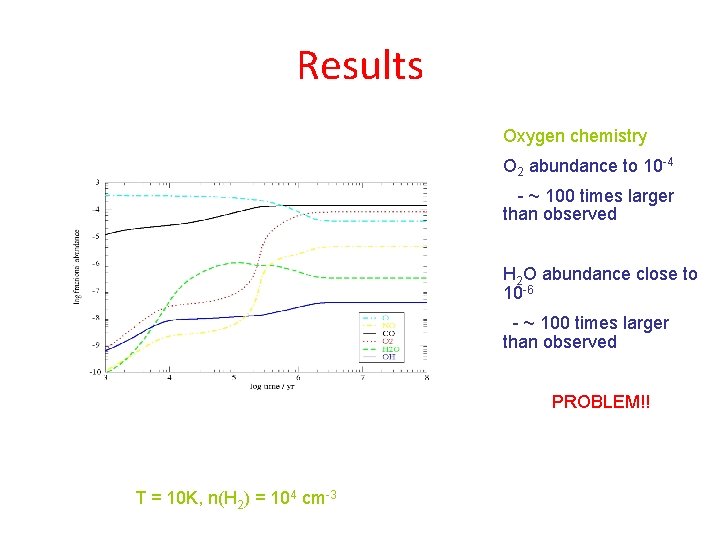
Results Oxygen chemistry O 2 abundance to 10 -4 - ~ 100 times larger than observed H 2 O abundance close to 10 -6 - ~ 100 times larger than observed PROBLEM!! T = 10 K, n(H 2) = 104 cm-3

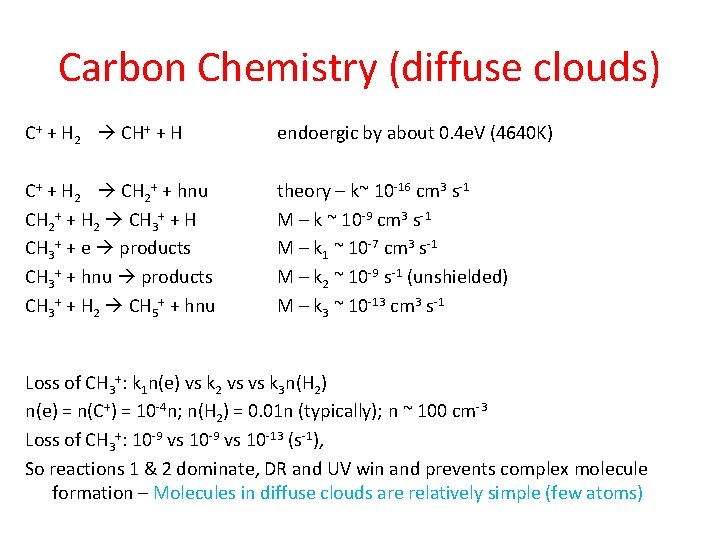
Carbon Chemistry (diffuse clouds) C+ + H 2 CH+ + H endoergic by about 0. 4 e. V (4640 K) C+ + H 2 CH 2+ + hnu CH 2+ + H 2 CH 3+ + H CH 3+ + e products CH 3+ + hnu products CH 3+ + H 2 CH 5+ + hnu theory – k~ 10 -16 cm 3 s-1 M – k ~ 10 -9 cm 3 s-1 M – k 1 ~ 10 -7 cm 3 s-1 M – k 2 ~ 10 -9 s-1 (unshielded) M – k 3 ~ 10 -13 cm 3 s-1 Loss of CH 3+: k 1 n(e) vs k 2 vs vs k 3 n(H 2) n(e) = n(C+) = 10 -4 n; n(H 2) = 0. 01 n (typically); n ~ 100 cm-3 Loss of CH 3+: 10 -9 vs 10 -13 (s-1), So reactions 1 & 2 dominate, DR and UV win and prevents complex molecule formation – Molecules in diffuse clouds are relatively simple (few atoms)

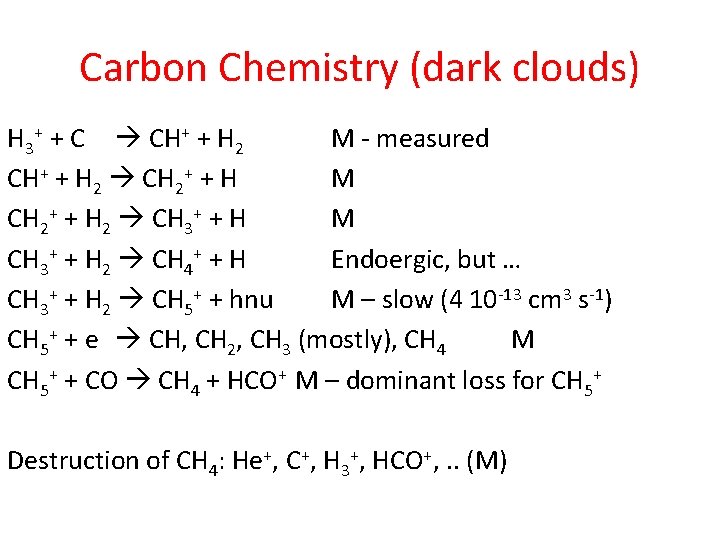
Carbon Chemistry (dark clouds) H 3+ + C CH+ + H 2 M - measured CH+ + H 2 CH 2+ + H M CH 2+ + H 2 CH 3+ + H M CH 3+ + H 2 CH 4+ + H Endoergic, but … CH 3+ + H 2 CH 5+ + hnu M – slow (4 10 -13 cm 3 s-1) CH 5+ + e CH, CH 2, CH 3 (mostly), CH 4 M CH 5+ + CO CH 4 + HCO+ M – dominant loss for CH 5+ Destruction of CH 4: He+, C+, H 3+, HCO+, . . (M)

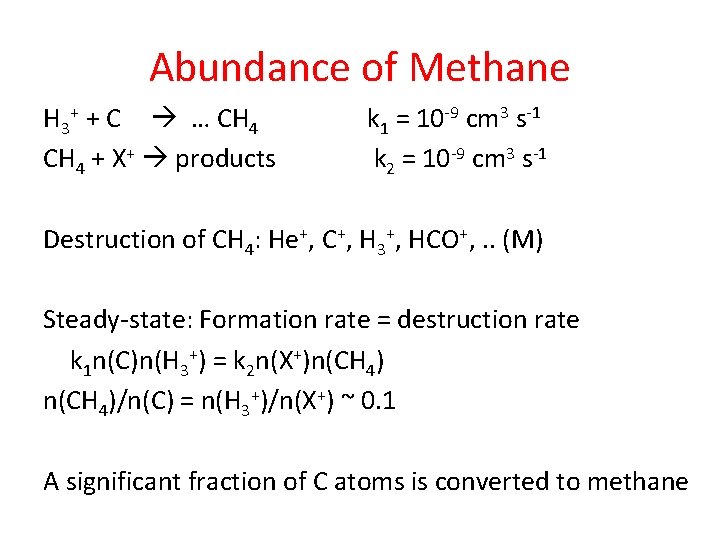
Abundance of Methane H 3+ + C … CH 4 + X+ products k 1 = 10 -9 cm 3 s-1 k 2 = 10 -9 cm 3 s-1 Destruction of CH 4: He+, C+, H 3+, HCO+, . . (M) Steady-state: Formation rate = destruction rate k 1 n(C)n(H 3+) = k 2 n(X+)n(CH 4)/n(C) = n(H 3+)/n(X+) ~ 0. 1 A significant fraction of C atoms is converted to methane

Formation of Organics Starts with proton transfer from H 3+ C + H 3+ CH+ + H 2 CH 2+ + H 2 CH 3+ + H 2 CH 5+ + hυ CH 5+ + CO CH 4 + HCO+ C+ + CH 4 C 2 H 2+ + H 2 C+ + CH 4 C 2 H 3+ + H

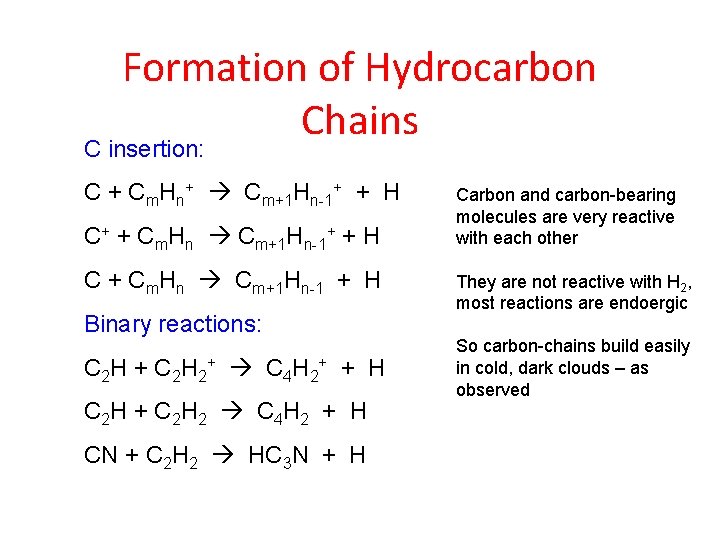
Formation of Hydrocarbon Chains C insertion: C + Cm. Hn+ Cm+1 Hn-1+ + H C+ + Cm. Hn Cm+1 Hn-1+ + H C + Cm. Hn Cm+1 Hn-1 + H Binary reactions: C 2 H + C 2 H 2+ C 4 H 2+ + H C 2 H + C 2 H 2 C 4 H 2 + H CN + C 2 H 2 HC 3 N + H Carbon and carbon-bearing molecules are very reactive with each other They are not reactive with H 2, most reactions are endoergic So carbon-chains build easily in cold, dark clouds – as observed

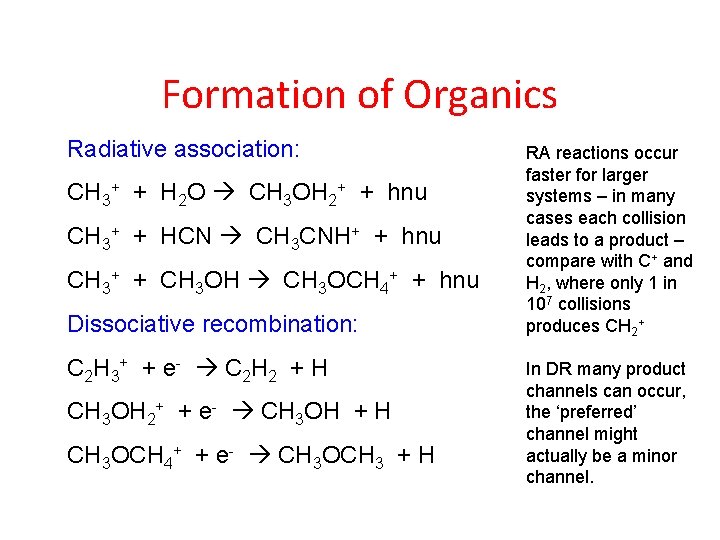
Formation of Organics Radiative association: CH 3+ + H 2 O CH 3 OH 2+ + hnu CH 3+ + HCN CH 3 CNH+ + hnu CH 3+ + CH 3 OH CH 3 OCH 4+ + hnu Dissociative recombination: C 2 H 3+ + e - C 2 H 2 + H CH 3 OH 2+ + e- CH 3 OH + H CH 3 OCH 4+ + e- CH 3 OCH 3 + H RA reactions occur faster for larger systems – in many cases each collision leads to a product – compare with C+ and H 2, where only 1 in 107 collisions produces CH 2+ In DR many product channels can occur, the ‘preferred’ channel might actually be a minor channel.