IonExchange Separation and Spectrophotometric Determination of Nickel and







- Slides: 8

Ion-Exchange Separation and Spectrophotometric Determination of Nickel and Cobalt By Doan-Phuong Dao

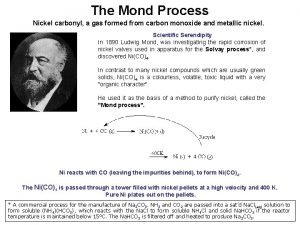
Background information This experiment had two major steps: 1)Separation of Ni and Co in the unknown sample by using an ion-exchange chromatography column with a strong base anion exchanger 2) Determination of the concentrations of Ni and Co in the unknown sample by using standard spectrophotometric procedures l

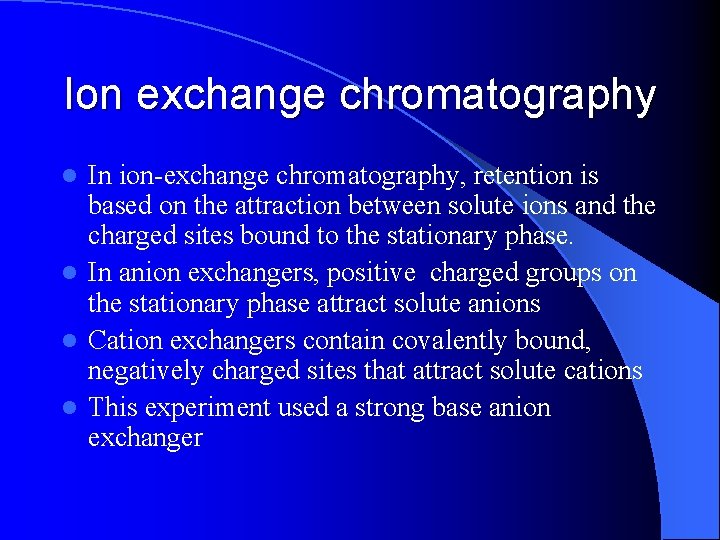
Ion exchange chromatography In ion-exchange chromatography, retention is based on the attraction between solute ions and the charged sites bound to the stationary phase. l In anion exchangers, positive charged groups on the stationary phase attract solute anions l Cation exchangers contain covalently bound, negatively charged sites that attract solute cations l This experiment used a strong base anion exchanger l

l In solution containing a large concentration of hydrochloric acid, Co was converted into complex anions (chlorides) Co. Cl 4 2 -, and was adsorbed by an anion resin. Ni was not converted into complex anions so that it was not adsorbed by such a resin. The separation of Nickel from Cobalt was easily effected. The cobalt is removed by washing the resin with 1 M HCl.

After separating from the mixture, Nickel was oxidized with bromine in ammonia solution and then treated with dimethylglyoxime. A wine-red or brown complex is formed. Cobalt was determined by conversion of the metal to the blue complex cobalt thiocyanate, Co(CNS)42 -. l 95% ethanol solution was used because both complexes were somewhat dissociated in water l The concentrations of Ni and Co were determined by measuring the absorbance at 455 nm (for Ni), and at 625 nm (for Co) with a spectrophotometer. l

The graphs of concentration vs. absorbance of Ni and Co standard solution were plotted to determine the line equations. l From the line equations , the absorbance of the Ni and Co in the unknown were plugged in to calculate the concentration of each metal in the sample l

Question l What is required when it comes to separating Nickel and Cobalt in the column?

Answer l Need 9 M HCl to bind to Cobalt and release Nickel l Need 1 M HCl to release the Cobalt from the column.