Investigating chirality Chemistry Review Vol 23 No 1
- Slides: 8

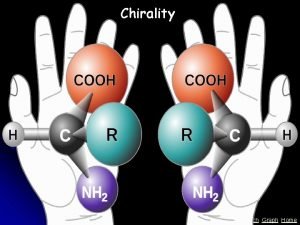
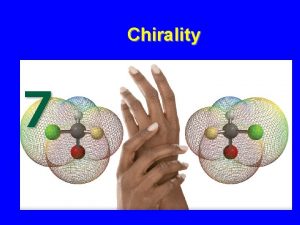
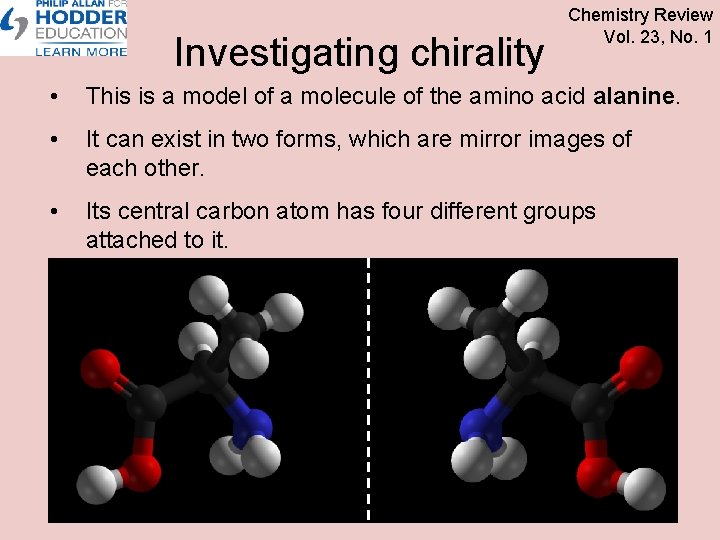
Investigating chirality Chemistry Review Vol. 23, No. 1 • This is a model of a molecule of the amino acid alanine. • It can exist in two forms, which are mirror images of each other. • Its central carbon atom has four different groups attached to it.

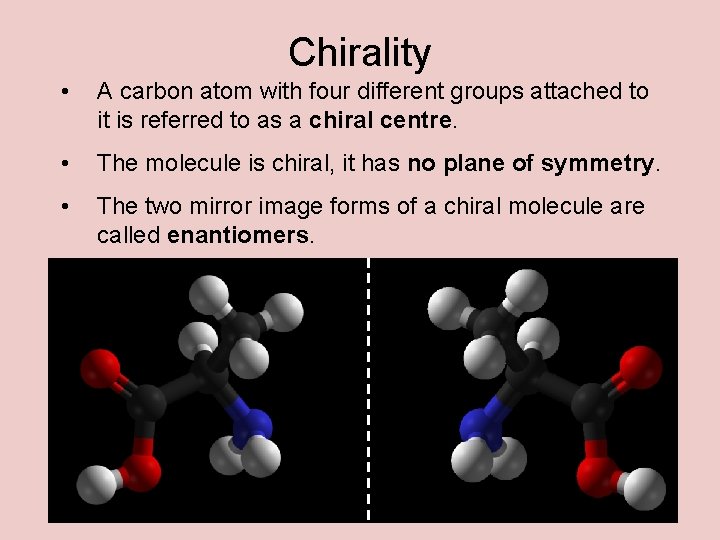
Chirality • A carbon atom with four different groups attached to it is referred to as a chiral centre. • The molecule is chiral, it has no plane of symmetry. • The two mirror image forms of a chiral molecule are called enantiomers.

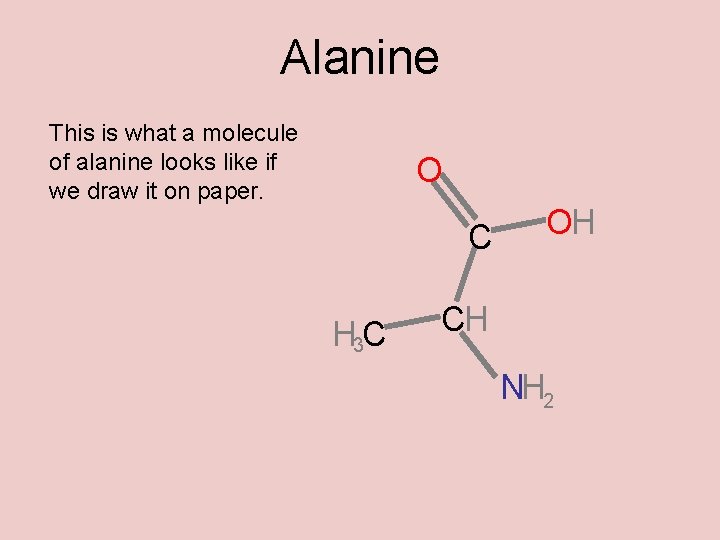
Alanine This is what a molecule of alanine looks like if we draw it on paper. O C H 3 C OH CH NH 2

Naming enantiomers • We need a way of identifying which form of a molecule is which. • There are different systems for naming molecules with a chiral centre. • Biologists tend to use L and D to label amino acids, as this makes most sense in a biological context. • The standard system used by chemists for all molecules with a chiral centre is the R/S system. • To identify whether a molecule is in the R form or the S form we use the Cahn–Ingold–Prelog rules.

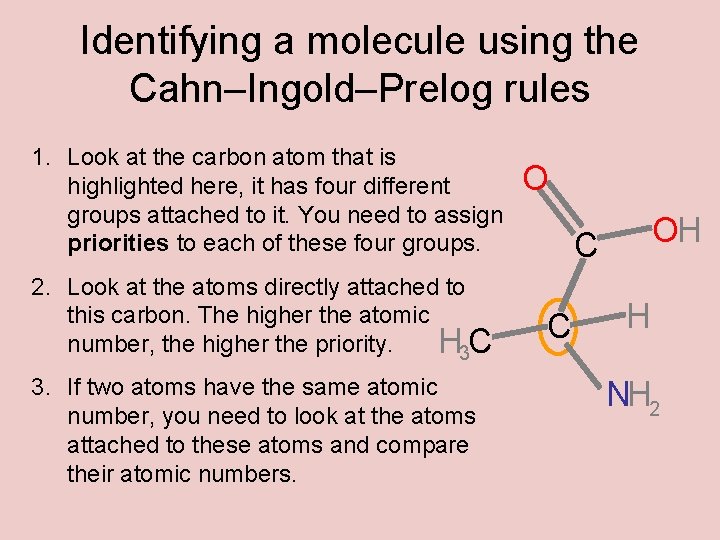
Identifying a molecule using the Cahn–Ingold–Prelog rules 1. Look at the carbon atom that is highlighted here, it has four different groups attached to it. You need to assign priorities to each of these four groups. 2. Look at the atoms directly attached to this carbon. The higher the atomic number, the higher the priority. H 3 C 3. If two atoms have the same atomic number, you need to look at the atoms attached to these atoms and compare their atomic numbers. O OH C C H NH 2

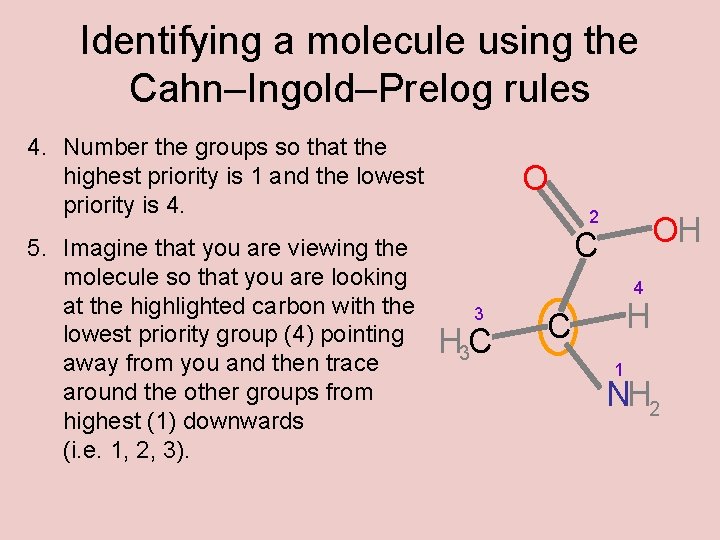
Identifying a molecule using the Cahn–Ingold–Prelog rules 4. Number the groups so that the highest priority is 1 and the lowest priority is 4. 5. Imagine that you are viewing the molecule so that you are looking at the highlighted carbon with the lowest priority group (4) pointing away from you and then trace around the other groups from highest (1) downwards (i. e. 1, 2, 3). O 2 OH C 4 3 H 3 C H C 1 NH 2

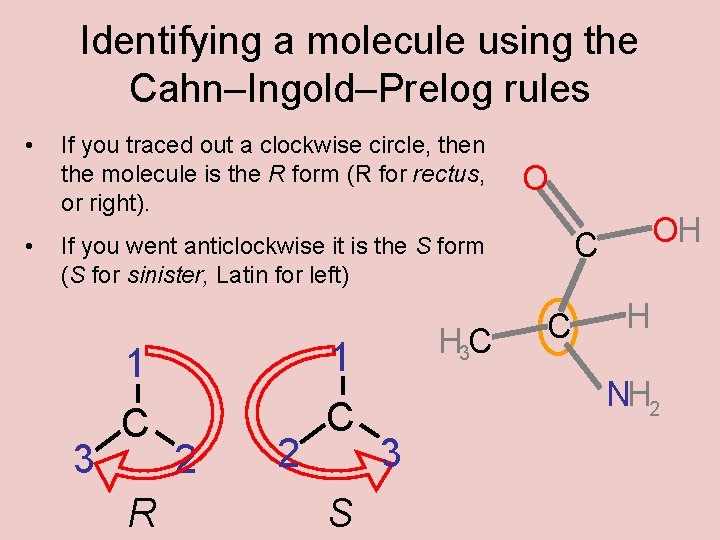
Identifying a molecule using the Cahn–Ingold–Prelog rules • • If you traced out a clockwise circle, then the molecule is the R form (R for rectus, or right). O 3 1 C R 2 2 1 C S H 3 C OH C If you went anticlockwise it is the S form (S for sinister, Latin for left) C H NH 2 3

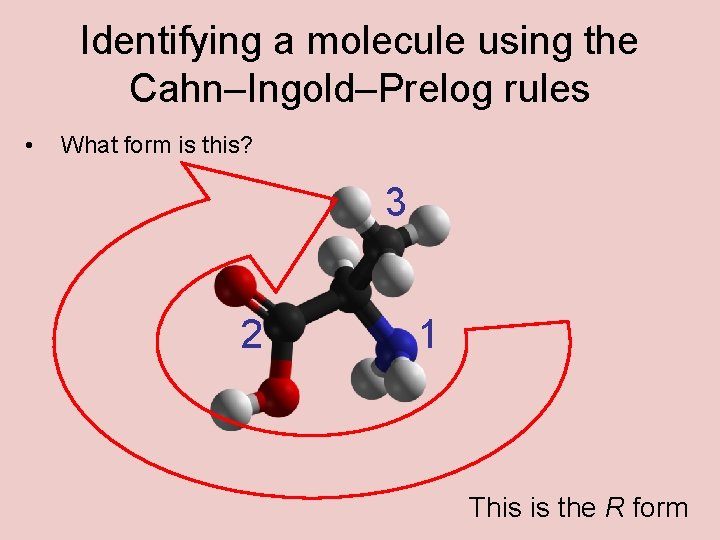
Identifying a molecule using the Cahn–Ingold–Prelog rules • What form is this? 3 2 1 This is the R form