Introduction to Food Science Chapter 6 Penfield and



















- Slides: 20

Introduction to Food Science Chapter 6 Penfield and Campbell 1990

Water The Universal Chemical

Exits in Three States Gas – Steam 2. Liquid – Water 3. Solid – Ice 1.

Heat Transfer is necessary for conversion Conversion from: Water Vapor to Liquid to Solid Heat must be removed = Exothermic

Liquid to Vapor Solid to Liquid Heat must be supplied = Endothermic

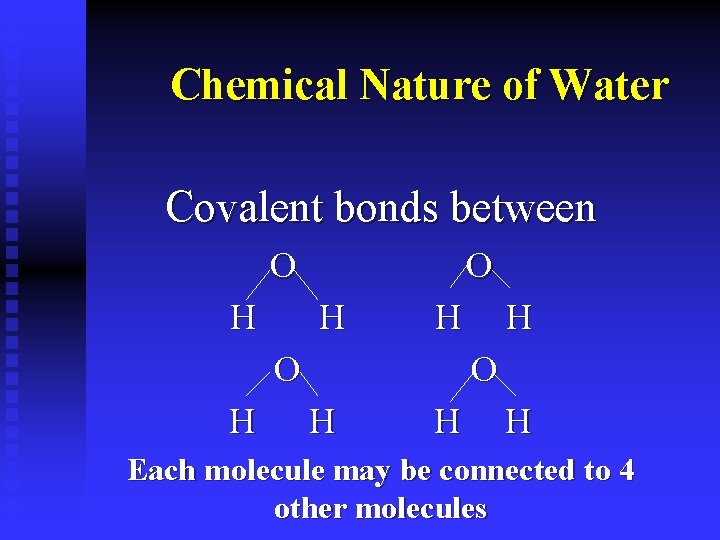
Chemical Nature of Water Covalent bonds between O H H

Chemical Nature of Water Covalent bonds between O H H H Each molecule may be connected to 4 other molecules

Natural Water is a solution.

Water -Trapped in capillaries of food -Water holding capacity Water activity aw

Water activity (a ) w 1. 00 – 0. 95 – 0. 90 – 0. 80 - 0. 70 - 0. 60 - 0. 50 0. 20 Fresh Meat, fruit, vegetables Processed cheese, baked goods Aged cheese, and jams Molasses, dried figs Dried Fruit and corn syrup Chocolate, honey, noodles Dried milk, dried vegetable

Food Dispersions Solutions: Homogeneous mixture of molecules and or ions of different substances one of which is water.

Food Dispersions Solutions: Sugar and Water Sodium Chloride and Water

Solubility is the amount of solute that can be dissolved in a given amount of solvent at a given temperature Concentration- is the amount of the solute dissolved in a specific solvent Saturated Solution - contains as much solute as can be dissolved at a given temperature.

Vapor Pressure - Pressure exerted by the gas above the liquid when equilibrium exists The greater the concentration of solute, greater the reduction in vapor pressure

Boiling Point- The temperature at which the vapor pressure equals the external pressure. Volatile solutes- Lower the boiling point because of their contribution to vaporization

Freezing point – Solutes despress the freezing point of water by disrupting structure

Osmotic Pressure – Osmosis is the passage of water molecules through a semipermeable membrane.

Colloidal Dispersions Sol - water is the continuous phase Gel - liquid-in-solid Emulsion – liquid-in-liquid


 St joseph church penfield
St joseph church penfield Penfield grade 5 answer key
Penfield grade 5 answer key Motor strip homunculus
Motor strip homunculus Penfield pavilion
Penfield pavilion Penfield robotics
Penfield robotics Think central science fusion
Think central science fusion My favorite subject in school is science
My favorite subject in school is science Unit 2 food food food
Unit 2 food food food Sequence of food chain
Sequence of food chain Forensic science chapter 1
Forensic science chapter 1 Food science an old but new subject
Food science an old but new subject Introduction to management science chapter 5 solutions
Introduction to management science chapter 5 solutions Introduction to management science chapter 1 solutions
Introduction to management science chapter 1 solutions Introduction to materials science for engineers chapter 10
Introduction to materials science for engineers chapter 10 Chapter 1 introduction to science
Chapter 1 introduction to science Chapter 1 introduction to earth science
Chapter 1 introduction to earth science Introduction to materials science for engineers chapter 10
Introduction to materials science for engineers chapter 10 The flow of food
The flow of food Natural science and social science similarities
Natural science and social science similarities Soft science definition
Soft science definition The science of food the nutrients and substances therein
The science of food the nutrients and substances therein



































